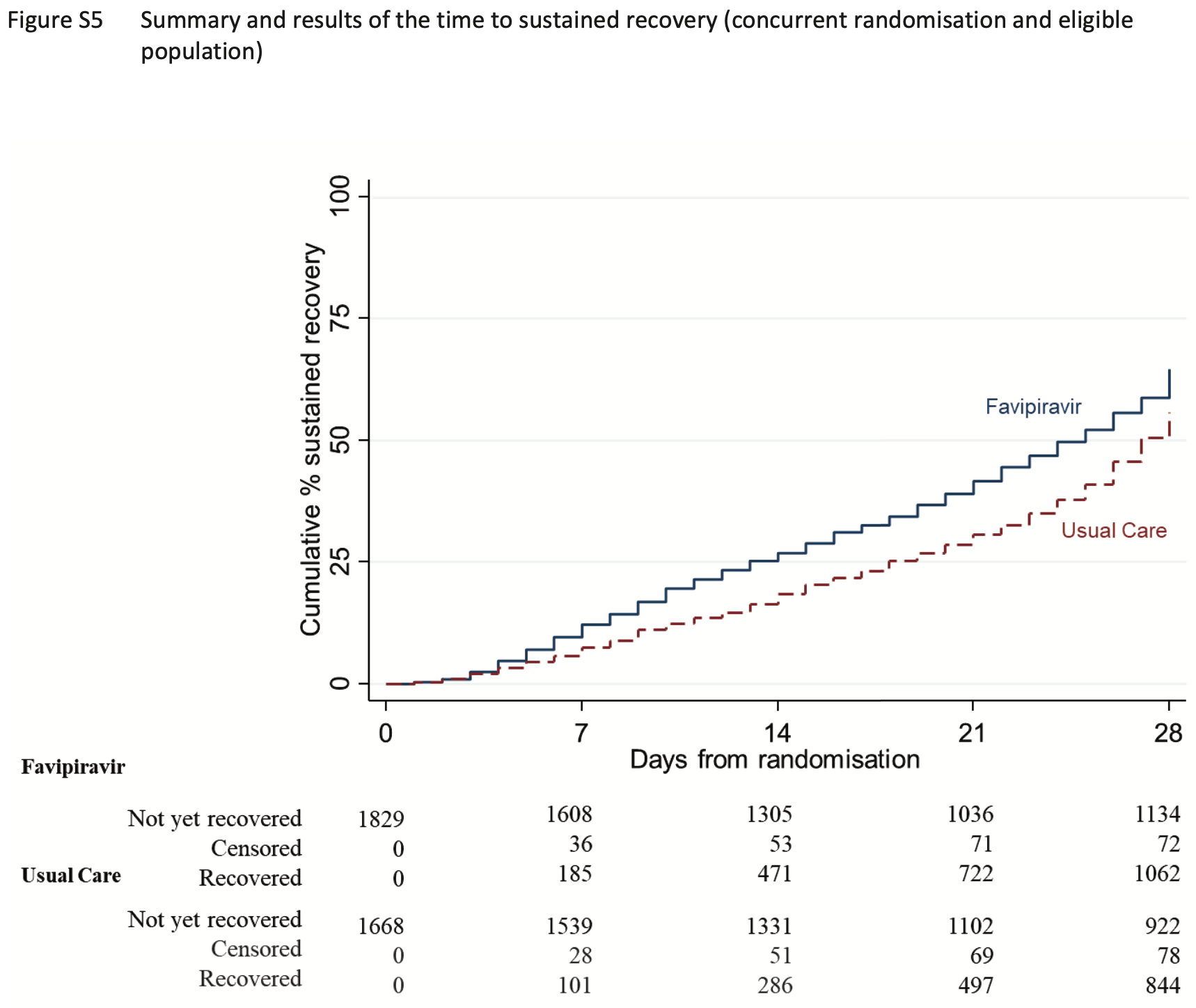
Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes
et al., Journal of Infection, doi:10.1016/j.jinf.2024.106248, PRINCIPLE, ISRCTN86534580, Aug 2024
RCT 3,622 (concurrent and eligible) COVID-19 outpatients in the UK showing significantly faster recovery with favipiravir, and significantly greater full recovery at 3, 6, and 12 months.
Authors note: "From 16 Dec 2021, a minority of extremely clinically vulnerable patients could also access antiviral treatment or a monoclonal antibody infusion". However, there is no information on treatments provided or procedures for determining eligibility. This change invalidates hospitalization/death data after 16 Dec 2021. Hospitalization/death events occured in a small minority of patients and are expected to be strongly biased towards the extremely clinically vulnerable patients. Patients randomized to usual care are more likely to obtain alternative treatment. During the trial extension period sotrovimab was the most common treatment, with paxlovid and molnupiravir also being used1. Sotrovimab showed very high efficacy during this period2,3. It is normal to provide details of other treatments used in cases like this, the lack of disclosure suggests that the data confirms alternative treatment use significantly biased the results.
Table 1 shows a median of 4 days delay from onset of symptoms, while Table S1 shows a mean of 5.1/5.0 days for the long-term followup patients (97% of patients) indicating a distribution skewed towards very late treatment.
c19early.org
PRINCIPLE trial selective reporting delays
Results for effective but politically inconvenient treatments were withheld for very long periods.
| Treatment patients | Duration | Results delay | |
|---|---|---|---|
| HCQ4 | 413 | 2 months | Over 5 years4 |
| Azithromycin5 | 540 | 6 months | 56 days6 |
| Budesonide7 | 1,073 | 4 months | 12 days8 |
| Doxycycline9 | 780 | 5 months | 42 days6 |
| Colchicine10 | 156 | 3 months | 120 days11 |
| Ivermectin12 | 2,157 | 14 months | 600 days (810 days from ~1,000 per arm enrollment) |
| Favipiravir13 | ~2,250 | 15 months | 780 days (1,000 days from ~1,000 per arm enrollment) |
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments14.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 86.3% lower, RR 0.14, p = 0.11, treatment 0 of 1,829 (0.0%), control 3 of 1,668 (0.2%), NNT 556, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of ICU admission, 191.1% higher, RR 2.91, p = 1.00, treatment 1 of 1,825 (0.1%), control 0 of 1,663 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of death/hospitalization, 1.0% lower, HR 0.99, p = 0.51, treatment 1,829, control 3,256, adjusted per study.
|
|
not fully recovered, 17.4% lower, RR 0.83, p = 0.003, treatment 350 of 1,582 (22.1%), control 378 of 1,412 (26.8%), NNT 22, day 365, Table 3.
|
|
not fully recovered, 12.2% lower, RR 0.88, p = 0.04, treatment 378 of 1,503 (25.1%), control 384 of 1,340 (28.7%), NNT 29, day 180, Table 3.
|
|
not fully recovered, 17.2% lower, RR 0.83, p < 0.001, treatment 418 of 1,507 (27.7%), control 459 of 1,370 (33.5%), NNT 17, day 90, Table 3.
|
|
ongoing persistent symptoms at 3, 6, 12 months, 29.0% lower, RR 0.71, p = 0.02, treatment 1,829, control 1,668, Table 3.
|
|
time to first reported recovery, 18.7% lower, HR 0.81, p < 0.001, treatment 1,829, control 3,256, adjusted per study, inverted to make HR<1 favor treatment, primary outcome.
|
|
early sustained recovery, 34.2% lower, RR 0.66, p < 0.001, treatment 1,828, control 1,666, adjusted per study, inverted to make RR<1 favor treatment.
|
|
sustained recovery, 24.8% lower, RR 0.75, p < 0.001, treatment 1,829, control 1,668, adjusted per study, inverted to make RR<1 favor treatment.
|
|
alleviation of all symptoms, 12.3% lower, RR 0.88, p < 0.001, treatment 1,562, control 1,407, adjusted per study, inverted to make RR<1 favor treatment.
|
|
sustained alleviation of all symptoms, 20.6% lower, RR 0.79, p < 0.001, treatment 1,562, control 1,407, adjusted per study, inverted to make RR<1 favor treatment.
|
|
initial reduction of severity, 23.1% lower, RR 0.77, p < 0.001, treatment 1,828, control 1,667, adjusted per study, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
england.nhs.uk, www.england.nhs.uk/statistics/wp-content/uploads/sites/2/2023/07/COVID-THERAPEUTICS-WEEKLY-PUBLICATION-week-ending-020723-V2.0-OCT-22-Onwards.xlsx.
2.
Behzad et al., Real world Effectiveness of Sotrovimab in Preventing COVID-19–related Hospitalisation or Death in Patients Infected with Omicron BA.2, Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.11.029.
3.
De Vito et al., What Is the Efficacy of Sotrovimab in Reducing Disease Progression and Death in People with COVID-19 during the Omicron Era? Answers from a Real-Life Study, Viruses, doi:10.3390/v15081757.
4.
Hobbs et al., The PRINCIPLE randomised controlled open label platform trial of hydroxychloroquine for treating COVID19 in community based patients at high risk, Scientific Reports, doi:10.1038/s41598-025-09275-6.
5.
Butler et al., Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet, doi:10.1016/S0140-6736(21)00461-X.
6.
nihr.ac.uk, www.nihr.ac.uk/news/principle-trial-finds-no-benefit-from-antibiotics-azithromycin-and-doxycycline-for-covid-19-patients/26680.
7.
Yu et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet, doi:10.1016/S0140-6736(21)01744-X.
8.
principletrial.org, www.principletrial.org/news/asthma-drug-budesonide-shortens-recovery-time-in-non-hospitalised-patients-with-covid-19.
10.
Dorward et al., Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, British Journal of General Practice, doi:10.3399/BJGP.2022.0083.
12.
Hayward et al., Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes, Journal of Infection, doi:10.1016/j.jinf.2024.106130.
Hobbs et al., 31 Aug 2024, Randomized Controlled Trial, United Kingdom, peer-reviewed, median age 54.1, 26 authors, study period 8 April, 2021 - 1 July, 2022, average treatment delay 5.1 days, trial ISRCTN86534580 (PRINCIPLE).
Contact: principle@phc.ox.ac.uk.
Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes
Journal of Infection, doi:10.1016/j.jinf.2024.106248
Background Evidence for the effect of favipiravir treatment of acute COVID-19 on recovery, hospital admissions and longer-term outcomes in community settings is limited.
Methods In this multicentre. open-label, multi-arm, adaptive platform randomised controlled trial participants aged ≥18 years in the community with a positive test for SARS-CoV-2 and symptoms lasting ≤14 days were randomised to: usual care; usual care plus favipiravir tablets (loading dose of 3600 mg in divided doses on day one, then 800 mg twice a day for four days); or, usual care plus other interventions. Co-primary endpoints were time to first selfreported recovery and hospitalisation/death related to COVID-19, within 28 days, analysed using Bayesian models. Recovery at six months was the primary longer-term outcome. Trial registration: ISRCTN86534580.
writing group critically revised the manuscript. The members of the PRINCIPLE Collaborative Group and their roles in the conduct of the trial are listed in the appendix.
Conflict of Interest statement Drs. Saville, Berry, Detry, Fitzgerald and Saunders report grants from The University of Oxford, for the Sponsor's grant from the UK NIHR, for statistical design and analyses for the
J o u r n a l P r e -p r o o f
Declaration of interests The authors declare the following financial interests/personal relationships which may be considered
References
Anand, Bradley, Mcauley, Clarke, Fool's gold? Why blinded trials are not always best, BMJ
Barsky, Saintfort, Rogers, Borus, Nonspecific medication side effects and the nocebo phenomenon, JAMA
Bosaeed, Alharbi, Alrehily, Bahlaq, Gaifer, Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial, Clinical Microbiology and Infection
Butler, Democratising the design and delivery of large-scale randomised, controlled clinical trials in primary care: A personal view, European Journal of General Practice
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, The Lancet
Butler, Yu, Dorward, Gbinigie, Hayward et al., Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet Respir Med
Cheema, Ali, Shahid, Ghafoor, Rehman, Efficacy and Safety of Favipiravir for the Treatment of COVID-19 Outpatients: A Systematic Review and Meta-analysis of Randomized Controlled Trials, Am J Ther
Dorward, Yu, Hayward, Saville, Gbinigie et al., Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial, Br J Gen Pract
Ford, Pragmatic Trials, N Engl J Med
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proceedings of the Japan Academy, Series B
Golan, Campos, Woolson, Cilla, Hanabergh et al., Favipiravir in Patients With Early Mild-to-moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial, Clin Infect Dis
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Hayward, Yu, Little, Gbinigie, Shanyinde et al., Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short-and longer-term outcomes, J Infect
Holubar, Subramanian, Purington, Hedlin, Bunning et al., Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial, Clin Infect Dis
Holubar, Subramanian, Purington, Hedlin, Bunning et al., Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial, Clinical Infectious Diseases
Little, Rumsby, Kelly, Watson, Moore et al., Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial, JAMA
Little, Williamson, Warner, Gould, Gantley et al., Open randomised trial of prescribing strategies in managing sore throat, BMJ
Lowe, Brown, Chowdhury, Davey, Yee et al., Favipiravir, lopinavirritonavir or combination therapy (FLARE): a randomised, double blind, 2x2 factorial placebocontrolled trial of early antiviral therapy in COVID-19, PLoS Med
Luvira, Schilling, Jittamala, Watson, Boyd et al., Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial, BMC Infect Dis
Mcmahon, Lau, Coldham, Roney, Hagenauer et al., Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial, EClinicalMedicine
Moustgaard, Clayton, Jones, Boutron, Jorgensen et al., Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study, BMJ
Ozgurbuz, Ensarioglu, Celik, Vatansever, Favipiravir Protects Enterocytes From Cell Death After Inflammatory Storm, Cureus
Patel, Dorward, Yu, Hobbs, Butler, Inclusion and diversity in the PRINCIPLE trial, The Lancet
Patel, Dorward, Yu, Hobbs, Butler, Inclusion and diversity in the PRINCIPLE trial, The Lancet
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic?, Journal of virus eradication
Png, Harris, Grabey, Hart, Jani et al., Cost-utility analysis of molnupiravir plus usual care versus usual care alone as early treatment for community-based adults with COVID-19 and increased risk of adverse outcomes in the UK PANORAMIC trial, Br J Gen Pract
Schober, Bossers, Schwarte, Statistical Significance Versus Clinical Importance of Observed Effect Sizes: What Do P Values and Confidence Intervals Really Represent?, Anesth Analg
Standing, Buggiotti, Guerra-Assuncao, Woodall, Ellis et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nat Commun
The, Infectious, Where are the long COVID trials?, Lancet Infect Dis
Thorpe, Zwarenstein, Oxman, Treweek, Furberg et al., A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers, J Clin Epidemiol
Topp, Østergaard, Søndergaard, Bech, The WHO-5 Well-Being Index: A Systematic Review of the Literature, Psychotherapy and Psychosomatics
Trial, Group, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
Verity, Okell, Dorigatti, Winskill, Whittaker et al., Estimates of the severity of coronavirus disease 2019: a model-based analysis, The Lancet Infectious Diseases
Wang, Anirudhan, Du, Cui, Rong, RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target, Journal of medical virology
Wartolowska, The nocebo effect as a source of bias in the assessment of treatment effects, F1000Res
Woodcock, Lavange, Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both, N Engl J Med
Yamamura, Matsuura, Nakagawa, Fukuoka, Domi et al., Effect of favipiravir and an anti-inflammatory strategy for COVID-19, Crit Care
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
DOI record:
{
"DOI": "10.1016/j.jinf.2024.106248",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2024.106248",
"alternative-id": [
"S0163445324001828"
],
"article-number": "106248",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2024.106248"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd on behalf of The British Infection Association."
}
],
"author": [
{
"affiliation": [],
"family": "Hobbs",
"given": "FD Richard",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gbinigie",
"given": "Oghenekome A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4842-9247",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shanyinde",
"given": "Milensu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Ly-Mee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3345-2826",
"affiliation": [],
"authenticated-orcid": false,
"family": "Harris",
"given": "Victoria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6072-1430",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dorward",
"given": "Jienchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayward",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saville",
"given": "Benjamin R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Nicholas S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Philip H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Nicholas PB",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahendra G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8093-7084",
"affiliation": [],
"authenticated-orcid": false,
"family": "Richards",
"given": "Duncan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hecke",
"given": "Oliver Van",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Detry",
"given": "Michelle A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Christina T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fitzgerald",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Jared",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0008-3801-1641",
"affiliation": [],
"authenticated-orcid": false,
"family": "Latimer-Bell",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Julie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7643-572X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ogburn",
"given": "Emma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7498-1276",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grabey",
"given": "Jenna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lusignan",
"given": "Simon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0619-1074",
"affiliation": [],
"authenticated-orcid": false,
"family": "Andersson",
"given": "Monique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Little",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Christopher C",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
29
]
],
"date-time": "2024-08-29T06:31:29Z",
"timestamp": 1724913089000
},
"deposited": {
"date-parts": [
[
2024,
8,
29
]
],
"date-time": "2024-08-29T06:31:50Z",
"timestamp": 1724913110000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "NIHR"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
30
]
],
"date-time": "2024-08-30T00:46:51Z",
"timestamp": 1724978811114
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 8,
"start": {
"date-parts": [
[
2024,
8,
9
]
],
"date-time": "2024-08-09T00:00:00Z",
"timestamp": 1723161600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445324001828?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445324001828?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "106248",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
8
]
]
},
"published-print": {
"date-parts": [
[
2024,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106248_bib1",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "10303",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2024.106248_bib2",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"article-title": "A review of the safety of favipiravir–a potential treatment in the COVID-19 pandemic?",
"author": "Pilkington",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "2",
"journal-title": "Journal of virus eradication",
"key": "10.1016/j.jinf.2024.106248_bib3",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"issue": "7",
"journal-title": "Proceedings of the Japan Academy, Series B",
"key": "10.1016/j.jinf.2024.106248_bib4",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1002/jmv.26264",
"article-title": "RNA‐dependent RNA polymerase of SARS‐CoV‐2 as a therapeutic target",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "300",
"issue": "1",
"journal-title": "Journal of medical virology",
"key": "10.1016/j.jinf.2024.106248_bib5",
"volume": "93",
"year": "2021"
},
{
"article-title": "Efficacy and Safety of Favipiravir for the Treatment of COVID-19 Outpatients: A Systematic Review and Meta-analysis of Randomized Controlled Trials",
"author": "Cheema",
"journal-title": "Am J Ther",
"key": "10.1016/j.jinf.2024.106248_bib6",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00440-1",
"article-title": "Where are the long COVID trials?",
"author": "The Lancet Infectious",
"doi-asserted-by": "crossref",
"first-page": "879",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jinf.2024.106248_bib7",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1056/NEJMra1510062",
"article-title": "Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "62",
"issue": "1",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106248_bib8",
"volume": "377",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(21)00461-X",
"article-title": "Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Principle Trial Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "1063",
"issue": "10279",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2024.106248_bib9",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00310-6",
"article-title": "Doxycycline for community treatment of suspected COVID-19 in people at high risk of adverse outcomes in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "1010",
"issue": "9",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.jinf.2024.106248_bib10",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3399/BJGP.2022.0083",
"article-title": "Colchicine for COVID-19 in the community (PRINCIPLE): a randomised, controlled, adaptive platform trial",
"author": "Dorward",
"doi-asserted-by": "crossref",
"first-page": "e446",
"issue": "720",
"journal-title": "Br J Gen Pract",
"key": "10.1016/j.jinf.2024.106248_bib11",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2024.106130",
"article-title": "Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"author": "Hayward",
"doi-asserted-by": "crossref",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2024.106248_bib12",
"year": "2024"
},
{
"key": "10.1016/j.jinf.2024.106248_bib13",
"unstructured": "Public Health England. COVID-19: investigation and initial clinical management of possible cases 2020 [Available from: 〈https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection〉."
},
{
"key": "10.1016/j.jinf.2024.106248_bib14",
"unstructured": "National Health Service. Symptoms of coronavirus 2021 [Available from: 〈https://www.nhs.uk/conditions/coronavirus-covid-19/symptoms/〉."
},
{
"DOI": "10.1016/S0140-6736(21)00945-4",
"article-title": "Inclusion and diversity in the PRINCIPLE trial",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "2251",
"issue": "10291",
"journal-title": "The Lancet",
"key": "10.1016/j.jinf.2024.106248_bib15",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00945-4",
"article-title": "Inclusion and diversity in the PRINCIPLE trial",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "2251",
"issue": "10291",
"journal-title": "The Lancet",
"key": "10.1016/j.jinf.2024.106248_bib16",
"volume": "397",
"year": "2021"
},
{
"author": "National Institute for Health and Care Excellence (NICE)",
"key": "10.1016/j.jinf.2024.106248_bib17",
"volume": "2020",
"year": "2020"
},
{
"key": "10.1016/j.jinf.2024.106248_bib18",
"unstructured": "National Institute for Health and Care Excellence. COVID-19 rapid guideline: Managing COVID-19 2022 [Available from: 〈https://app.magicapp.org/#/guideline/L4Qb5n/section/nBMk69〉."
},
{
"key": "10.1016/j.jinf.2024.106248_bib19",
"unstructured": "The United Kingdom Government. Coronavirus (COVID-19) in the UK 2020 [updated February 12, 2021. Available from: 〈https://coronavirus.data.gov.uk/〉."
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"article-title": "Estimates of the severity of coronavirus disease 2019: a model-based analysis",
"author": "Verity",
"doi-asserted-by": "crossref",
"first-page": "669",
"issue": "6",
"journal-title": "The Lancet Infectious Diseases",
"key": "10.1016/j.jinf.2024.106248_bib20",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1159/000376585",
"article-title": "The WHO-5 Well-Being Index: A Systematic Review of the Literature",
"author": "Topp",
"doi-asserted-by": "crossref",
"first-page": "167",
"issue": "3",
"journal-title": "Psychotherapy and Psychosomatics",
"key": "10.1016/j.jinf.2024.106248_bib21",
"volume": "84",
"year": "2015"
},
{
"DOI": "10.1371/journal.pmed.1004120",
"article-title": "Favipiravir, lopinavir-ritonavir or combination therapy (FLARE): a randomised, double blind, 2x2 factorial placebo-controlled trial of early antiviral therapy in COVID-19",
"author": "Lowe",
"doi-asserted-by": "crossref",
"issue": "10",
"journal-title": "PLoS Med",
"key": "10.1016/j.jinf.2024.106248_bib22",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac312",
"article-title": "Favipiravir for treatment of outpatients with asymptomatic or uncomplicated COVID-19: a double-blind randomized, placebo-controlled, phase 2 trial",
"author": "Holubar",
"doi-asserted-by": "crossref",
"journal-title": "Clinical Infectious Diseases",
"key": "10.1016/j.jinf.2024.106248_bib23",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"article-title": "Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial",
"author": "Bosaeed",
"doi-asserted-by": "crossref",
"first-page": "602",
"issue": "4",
"journal-title": "Clinical Microbiology and Infection",
"key": "10.1016/j.jinf.2024.106248_bib24",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac712",
"article-title": "Favipiravir in Patients With Early Mild-to-moderate Coronavirus Disease 2019 (COVID-19): A Randomized Controlled Trial",
"author": "Golan",
"doi-asserted-by": "crossref",
"first-page": "e10",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2024.106248_bib25",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2022.101703",
"article-title": "Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial",
"author": "McMahon",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.jinf.2024.106248_bib26",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac312",
"article-title": "Favipiravir for Treatment of Outpatients With Asymptomatic or Uncomplicated Coronavirus Disease 2019: A Double-Blind, Randomized, Placebo-Controlled, Phase 2 Trial",
"author": "Holubar",
"doi-asserted-by": "crossref",
"first-page": "1883",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2024.106248_bib27",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1016/j.jclinepi.2008.12.011",
"article-title": "A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers",
"author": "Thorpe",
"doi-asserted-by": "crossref",
"first-page": "464",
"issue": "5",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/j.jinf.2024.106248_bib28",
"volume": "62",
"year": "2009"
},
{
"DOI": "10.1056/NEJMra1510059",
"article-title": "Pragmatic Trials",
"author": "Ford",
"doi-asserted-by": "crossref",
"first-page": "454",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2024.106248_bib29",
"volume": "375",
"year": "2016"
},
{
"DOI": "10.1001/jama.287.5.622",
"article-title": "Nonspecific medication side effects and the nocebo phenomenon",
"author": "Barsky",
"doi-asserted-by": "crossref",
"first-page": "622",
"issue": "5",
"journal-title": "JAMA",
"key": "10.1016/j.jinf.2024.106248_bib30",
"volume": "287",
"year": "2002"
},
{
"article-title": "The nocebo effect as a source of bias in the assessment of treatment effects",
"author": "Wartolowska",
"first-page": "5",
"journal-title": "F1000Res",
"key": "10.1016/j.jinf.2024.106248_bib31",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1186/s12879-023-08835-3",
"article-title": "Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial",
"author": "Luvira",
"doi-asserted-by": "crossref",
"first-page": "89",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jinf.2024.106248_bib32",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1186/s13054-020-03137-5",
"article-title": "Effect of favipiravir and an anti-inflammatory strategy for COVID-19",
"author": "Yamamura",
"doi-asserted-by": "crossref",
"first-page": "413",
"issue": "1",
"journal-title": "Crit Care",
"key": "10.1016/j.jinf.2024.106248_bib33",
"volume": "24",
"year": "2020"
},
{
"article-title": "Favipiravir Protects Enterocytes From Cell Death After Inflammatory Storm",
"author": "Ozgurbuz",
"issue": "10",
"journal-title": "Cureus",
"key": "10.1016/j.jinf.2024.106248_bib34",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1136/bmj.314.7082.722",
"article-title": "Open randomised trial of prescribing strategies in managing sore throat",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "722",
"issue": "7082",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2024.106248_bib35",
"volume": "314",
"year": "1997"
},
{
"DOI": "10.1001/jama.293.24.3029",
"article-title": "Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "3029",
"issue": "24",
"journal-title": "JAMA",
"key": "10.1016/j.jinf.2024.106248_bib36",
"volume": "293",
"year": "2005"
},
{
"DOI": "10.1136/bmj.l6802",
"article-title": "Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study",
"author": "Moustgaard",
"doi-asserted-by": "crossref",
"first-page": "l6802",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2024.106248_bib37",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1136/bmj.l6228",
"article-title": "Fool's gold? Why blinded trials are not always best.",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "l6228",
"journal-title": "BMJ",
"key": "10.1016/j.jinf.2024.106248_bib38",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1213/ANE.0000000000002798",
"article-title": "Statistical Significance Versus Clinical Importance of Observed Effect Sizes: What Do P Values and Confidence Intervals Really Represent?",
"author": "Schober",
"doi-asserted-by": "crossref",
"first-page": "1068",
"issue": "3",
"journal-title": "Anesth Analg",
"key": "10.1016/j.jinf.2024.106248_bib39",
"volume": "126",
"year": "2018"
},
{
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC)",
"author": "Butler",
"journal-title": "an open-label, platform-adaptive randomised controlled trial. The Lancet.",
"key": "10.1016/j.jinf.2024.106248_bib40",
"year": "2022"
},
{
"article-title": "Democratising the design and delivery of large-scale randomised, controlled clinical trials in primary care: A personal view",
"author": "Butler",
"journal-title": "European Journal of General Practice",
"key": "10.1016/j.jinf.2024.106248_bib41",
"year": "2023"
},
{
"DOI": "10.3399/BJGP.2023.0444",
"article-title": "Cost-utility analysis of molnupiravir plus usual care versus usual care alone as early treatment for community-based adults with COVID-19 and increased risk of adverse outcomes in the UK PANORAMIC trial",
"author": "Png",
"doi-asserted-by": "crossref",
"journal-title": "Br J Gen Pract",
"key": "10.1016/j.jinf.2024.106248_bib42",
"year": "2024"
},
{
"DOI": "10.1038/s41467-024-45641-0",
"article-title": "Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients",
"author": "Standing",
"doi-asserted-by": "crossref",
"first-page": "1652",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.jinf.2024.106248_bib43",
"volume": "15",
"year": "2024"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445324001828"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}