In-hospital mortality outcomes of favipiravir in patients with moderate to severe COVID-19 infection: An emulated target trial using real-world data from the largest field hospital in Thailand
et al., PLOS One, doi:10.1371/journal.pone.0324903, Jun 2025
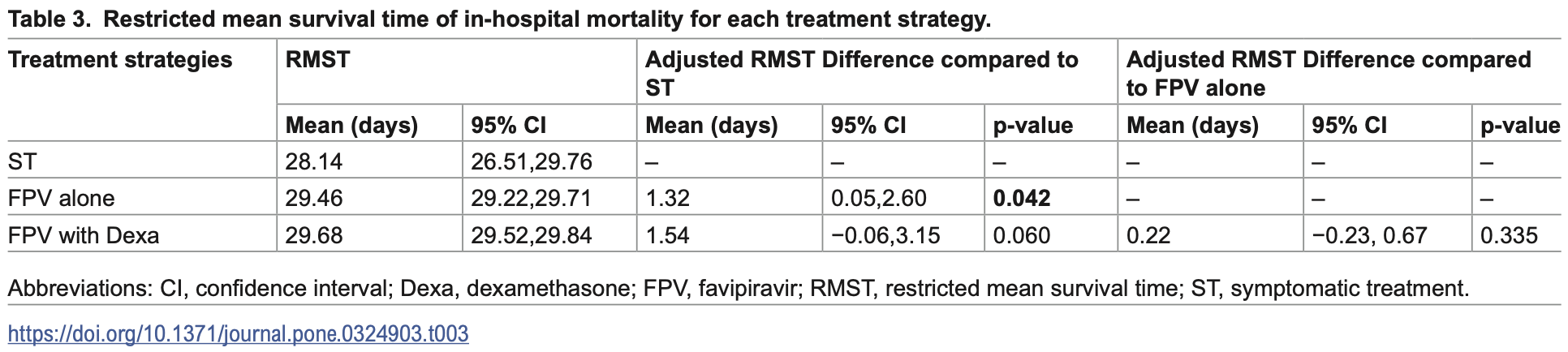
Retrospective 3,193 moderate to severe COVID-19 patients in Thailand showing modest survival benefits with favipiravir. This emulated target trial found that favipiravir alone increased restricted mean survival time by 1.32 days (p=0.042) compared to symptomatic treatment, while favipiravir combined with dexamethasone showed a marginally significant benefit (p=0.060). The benefits were more pronounced in patients with hypoxia, pneumonia, and male patients. Authors employed cloning-censoring techniques to minimize immortal time bias and baseline confounding.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
survival time, 4.5% lower, relative time 0.96, p = 0.004, treatment mean 29.46 (±3.6) n=828, control mean 28.14 (±8.66) n=109.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Lumkul et al., 4 Jun 2025, retrospective, Thailand, peer-reviewed, 5 authors, study period 14 May, 2021 - 20 September, 2021.
Contact: thot_kwan@hotmail.com, phichayutphinyo@gmail.com.
In-hospital mortality outcomes of favipiravir in patients with moderate to severe COVID-19 infection: An emulated target trial using real-world data from the largest field hospital in Thailand
PLOS One, doi:10.1371/journal.pone.0324903
Background Favipiravir, an antiviral agent, has been widely used to treat COVID-19 due to its potential mechanism of action, despite limited evidence of its efficacy in moderate to severe cases.
Aim This study aimed to evaluate the efficacy of favipiravir in improving in-hospital mortality outcomes among patients with moderate to severe COVID-19 through an emulation of a target trial.
Methods We emulated a target trial using observational data from Bussarakham field hospital, Thailand between May 14 and September 20, 2021. Patients were categorized into three groups: those receiving favipiravir with dexamethasone (FPV with Dexa), favipiravir alone (FPV), and symptomatic treatment (ST). In-hospital mortality within 30 days was the primary outcome.
Results From 18,184 patients admitted to the hospital, a total of 3,193 moderate to severe COVID-19 cases were included. Of these, 2,256 (70.65%) received FPV with Dexa,
References
Agrawal, Raju, Udwadia, Favipiravir: A new and emerging antiviral option in COVID-19, Med J Armed Forces India, doi:10.1016/j.mjafi.2020.08.004
Al-Muhsen, Ns, Sharif-Askari, Basamh, Alyounes et al., Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia, Front Med, doi:10.3389/fmed.2022.826247
Angermair, Hardenberg, Rubarth, Balzer, Akbari et al., In-hospital survival of critically ill COVID-19 patients treated with glucocorticoids: a multicenter real-world data study, Sci Rep, doi:10.1038/s41598-024-62302-w
Bosaeed, Alharbi, Altayib, Albayat, Alharbi, Favipiravir and Hydroxychloroquine Combination Therapy in Patients with Moderate to Severe COVID-19 (FACCT Trial): An Open-Label, Multicenter, Randomized, Controlled Trial, Infect Dis Ther, doi:10.1007/s40121-021-00496-6
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Eng
Chavalertsakul, Sutherasan, Petnak, Thammavaranucupt, Kirdlarp et al., Remdesivir versus Favipiravir in Hospitalized Patients with Moderate to Severe COVID-19 Pneumonia: A Propensity Score-Matched Retrospective Cohort Study, Int J Gen Med, doi:10.2147/IJGM.S457198
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial, Front Pharmacol, doi:10.3389/fphar.2021.683296
Doi, Ishihara, Banno, Ando, Kondo et al., Favipiravir for symptomatic COVID-19: A nationwide observational cohort study, J Infect Chemother, doi:10.1016/j.jiac.2022.10.008
Funk, Westreich, Wiesen, Stürmer, Brookhart et al., Doubly robust estimation of causal effects, Am J Epidemiol, doi:10.1093/aje/kwq439
Harris, Taylor, Minor, Elliott, Fernandez et al., The REDCap consortium: Building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:10.1016/j.jbi.2008.08.010
Harvey, Molecular mechanisms of dexamethasone actions in COVID-19: Ion channels and airway surface liquid dynamics, Steroids, doi:10.1016/j.steroids.2023.109348
Health, Nio, COVID-19 Treatment Guidelines Panel
Hernán, Sauer, Hernández-Díaz, Platt, Shrier, Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses, J Clin Epidemiol
Hobbs, Gbinigie-Thompson, Shanyinde, Yu, Harris et al., Favipiravir for COVID-19 in adults in the community in PRIN-CIPLE, an open-label, randomised, controlled, adaptive platform trial of short-and longer-term outcomes, J Infect, doi:10.1016/j.jinf.2024.106248
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with covid-19, N Engl J Med
Janiaud, Axfors, Ioannidis, Hemkens, Recruitment and Results Reporting of COVID-19 Randomized Clinical Trials Registered in the First 100 Days of the Pandemic, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.0330
Kamali, Sarmadian, Mahmoodiyeh, Valibeik, Farmani et al., Evaluation of the effect of favipiravir on patients with COVID-19, J Family Med Prim Care, doi:10.4103/jfmpc.jfmpc_1058_22
Kim, Uno, Wei, Restricted Mean Survival Time as a Measure to Interpret Clinical Trial Results, JAMA Cardiol, doi:10.1001/jamacardio.2017.2922
Kowarik, Templ, Imputation with the R Package VIM, J Stat Soft, doi:10.18637/jss.v074.i07
Kuri-Ayache, Rivera-Cavazos, Pérez-Castillo, Santos-Macías, González-Cantú et al., Viral load and its relationship with the inflammatory response and clinical outcomes in hospitalization of patients with COVID-19, Front Immunol
Lou, Liu, Yao, Hu, Su et al., Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial, Eur J Pharm Sci
Maringe, Benitez Majano, Exarchakou, Smith, Rachet et al., Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data, Int J Epidemiol, doi:10.1093/ije/dyaa057
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Mourad, Thibault, Holland, Yang, Young et al., Dexamethasone for Inpatients With COVID-19 in a National Cohort, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.8516
Mutair, Shamou, Alhumaid, Layqah, Ahmed et al., Overview of clinical outcome and therapeutic effectiveness of favipiravir in patients with COVID-19 admitted to intensive care unit, Riyadh, Saudi Arabia, J Infect Public Health
Nation, Locally made Favipiravir tablets used for Covid treatment get FDA approval
Neyton, Patel, Sarma, Ucsf Comet Consortium, Willmore et al., Distinct pulmonary and systemic effects of dexamethasone in severe COVID-19, Nat Commun, doi:10.1038/s41467-024-49756-2
Organization, Clinical management of COVID-19: living guideline
Peng, Peng, Yuan, Wang, Zhao et al., Structural Basis of SARS-CoV-2 Polymerase Inhibition by Favipiravir, Innovation (Camb), doi:10.1016/j.xinn.2021.100080
Qomara, Primanissa, Amalia, Purwadi, Zakiyah, Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review, Int J Gen Med, doi:10.2147/IJGM.S332458
Rattanachaikunsopon, Third Wave of COVID-19 in Thailand
Rattanaumpawan, Jirajariyavej, Lerdlamyong, Palavutitotai, Saiyarin, Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand, Antibiotics, doi:10.3390/antibiotics11060805
Sawanpanyalert, Sangsayunh, Putcharoen, Manosuthi, Intalapaporn et al., Assessment of outcomes following implementation of antiviral treatment guidelines for covid-19 during the first wave in thailand, Southeast Asian J Trop Med Public Health
Shah, Orton, Grinsztejn, Donaldson, Ramírez et al., Favipiravir in patients hospitalised with COVID-19 (PIO-NEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care, Lancet Respir Med, doi:10.1016/S2213-2600(22)00412-X
Shenoy, Munjal, Youha, Alghounaim, Almazeedi et al., -blinded, placebo-controlled trial
Shiraki, Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol Ther, doi:10.1016/j.pharmthera.2020.107512
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2117092
Siripongboonsitti, Muadchimkaew, Tawinprai, Issaranon, Meepholkij et al., Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV), Sci Rep, doi:10.1038/s41598-023-42195-x
Sohrabi, Alsafi, Neill, Khan, Kerwan et al., World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19), Int J Surg, doi:10.1016/j.ijsu.2020.02.034
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107522
Srisubat, Thanasitthichai, Kongsaengdao, Maneeton, Maneeton et al., Effectiveness of favipiravir monotherapy in the treatment of COVID-19: real world data analysis from Thailand, Lancet Reg Health Southeast Asia, doi:10.1016/j.lansea.2023.100166
Sulaiman, Aljuhani, Korayem, Altebainawi, Alfaifi et al., When antivirals backfire: An evaluation of favipiravir's clinical outcomes in critically ill patients with COVID-19: A multicenter cohort study, J Infect Public Health, doi:10.1016/j.jiph.2023.06.011
Svanberg, Macpherson, Zucco, Agius, Faitova et al., Early stimulated immune responses predict clinical disease severity in hospitalized COVID-19 patients, Commun Med (Lond), doi:10.1038/s43856-022-00178-5
Tabarsi, Vahidi, Saffaei, Hashemian, Jammati et al., Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form, Iran J Pharm Res, doi:10.22037/ijpr.2021.115510.15401
Tantipasawasin, Sars-cov-2 delta variant: The 2nd wave covid-19 pandemic in thailand, Chonburi Hosp J
Twohig, Nyberg, Zaidi, Thelwall, Sinnathamby et al., Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00475-8
DOI record:
{
"DOI": "10.1371/journal.pone.0324903",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0324903",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Favipiravir, an antiviral agent, has been widely used to treat COVID-19 due to its potential mechanism of action, despite limited evidence of its efficacy in moderate to severe cases.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Aim</jats:title>\n<jats:p>This study aimed to evaluate the efficacy of favipiravir in improving in-hospital mortality outcomes among patients with moderate to severe COVID-19 through an emulation of a target trial.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Methods</jats:title>\n<jats:p>We emulated a target trial using observational data from Bussarakham field hospital, Thailand between May 14 and September 20, 2021. Patients were categorized into three groups: those receiving favipiravir with dexamethasone (FPV with Dexa), favipiravir alone (FPV), and symptomatic treatment (ST). In-hospital mortality within 30 days was the primary outcome.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Results</jats:title>\n<jats:p>From 18,184 patients admitted to the hospital, a total of 3,193 moderate to severe COVID-19 cases were included. Of these, 2,256 (70.65%) received FPV with Dexa, 828 (25.93%) received FPV, and 109 (3.41%) received ST. The restricted mean survival times were 29.68 days (95% CI: 29.52, 29.84) for FPV with Dexa, 29.46 days (95% CI: 29.22, 29.71) for FPV, and 28.14 days (95% CI: 26.51, 29.76) for ST. Only FPV showed marginally significant difference when compared to ST. However, there was a trend in prolonging survival time in FPV with Dexa group, and the results were more pronounced in severe and hypoxic patients. </jats:p>\n</jats:sec>\n<jats:sec id=\"sec005\">\n<jats:title>Conclusion</jats:title>\n<jats:p>Our emulated target trial suggests favipiravir, especially with dexamethasone, offers a modest survival benefit in moderate to severe COVID-19, particularly in hypoxic patients. It supports favipiravir as a practical antiviral in settings where other antivirals are not available. Further randomized controlled studies are needed to confirm its role, alongside standard corticosteroid therapy.</jats:p>\n</jats:sec>",
"author": [
{
"affiliation": [],
"family": "Lumkul",
"given": "Lalita",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tanasombatkul",
"given": "Krittai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nitikaroon",
"given": "Phongsak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morasert",
"given": "Thotsaporn",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8543-6254",
"affiliation": [],
"authenticated-orcid": true,
"family": "Phinyo",
"given": "Phichayut",
"sequence": "additional"
}
],
"container-title": "PLOS One",
"container-title-short": "PLoS One",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T18:07:39Z",
"timestamp": 1749060459000
},
"deposited": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T18:07:51Z",
"timestamp": 1749060471000
},
"editor": [
{
"affiliation": [],
"family": "Bertagnin",
"given": "Chiara",
"sequence": "first"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T04:15:45Z",
"timestamp": 1749096945611,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2025,
6,
4
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2025,
6,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T00:00:00Z",
"timestamp": 1748995200000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0324903",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0324903",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2025,
6,
4
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
4
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1016/j.ijsu.2020.02.034",
"article-title": "World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19)",
"author": "C Sohrabi",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Int J Surg",
"key": "pone.0324903.ref001",
"volume": "76",
"year": "2020"
},
{
"key": "pone.0324903.ref002",
"year": "2024"
},
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"article-title": "Favipiravir: A new and emerging antiviral option in COVID-19",
"author": "U Agrawal",
"doi-asserted-by": "crossref",
"first-page": "370",
"issue": "4",
"journal-title": "Med J Armed Forces India",
"key": "pone.0324903.ref003",
"volume": "76",
"year": "2020"
},
{
"author": "Department of Medical Services T",
"key": "pone.0324903.ref004",
"volume-title": "COVID-19 treatment guidelines",
"year": "2020"
},
{
"article-title": "Assessment of outcomes following implementation of antiviral treatment guidelines for covid-19 during the first wave in thailand",
"author": "N Sawanpanyalert",
"first-page": "572",
"issue": "4",
"journal-title": "Southeast Asian J Trop Med Public Health",
"key": "pone.0324903.ref005",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1016/j.pharmthera.2020.107512",
"article-title": "Favipiravir, an anti-influenza drug against life-threatening RNA virus infections",
"author": "K Shiraki",
"doi-asserted-by": "crossref",
"first-page": "107512",
"journal-title": "Pharmacol Ther",
"key": "pone.0324903.ref006",
"volume": "209",
"year": "2020"
},
{
"key": "pone.0324903.ref007",
"unstructured": "Rattanachaikunsopon PPP. Third Wave of COVID-19 in Thailand."
},
{
"article-title": "Sars-cov-2 delta variant: The 2nd wave covid-19 pandemic in thailand",
"author": "S Tantipasawasin",
"first-page": "81",
"issue": "2",
"journal-title": "Chonburi Hosp J",
"key": "pone.0324903.ref008",
"volume": "46",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(21)00475-8",
"article-title": "Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study",
"author": "KA Twohig",
"doi-asserted-by": "crossref",
"first-page": "35",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "pone.0324903.ref009",
"volume": "22",
"year": "2022"
},
{
"author": "Department of Medical Services T",
"key": "pone.0324903.ref010",
"volume-title": "COVID-19 treatment guidelines",
"year": "2023"
},
{
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Q Cai",
"first-page": "1192",
"issue": "10",
"journal-title": "Eng (Beijing)",
"key": "pone.0324903.ref011",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2022.10.008",
"article-title": "Favipiravir for symptomatic COVID-19: A nationwide observational cohort study",
"author": "Y Doi",
"doi-asserted-by": "crossref",
"first-page": "150",
"issue": "2",
"journal-title": "J Infect Chemother",
"key": "pone.0324903.ref012",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1007/s40121-021-00496-6",
"article-title": "Favipiravir and Hydroxychloroquine Combination Therapy in Patients with Moderate to Severe COVID-19 (FACCT Trial): An Open-Label, Multicenter, Randomized, Controlled Trial",
"author": "M Bosaeed",
"doi-asserted-by": "crossref",
"first-page": "2291",
"issue": "4",
"journal-title": "Infect Dis Ther",
"key": "pone.0324903.ref013",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s43856-022-00178-5",
"article-title": "Early stimulated immune responses predict clinical disease severity in hospitalized COVID-19 patients",
"author": "R Svanberg",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Commun Med (Lond)",
"key": "pone.0324903.ref014",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"author": "P Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "pone.0324903.ref015",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"article-title": "Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study",
"author": "R Sirijatuphat",
"doi-asserted-by": "crossref",
"first-page": "2197",
"issue": "1",
"journal-title": "Emerg Microbes Infect",
"key": "pone.0324903.ref016",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41598-023-42195-x",
"article-title": "Favipiravir treatment in non-severe COVID-19: promising results from multicenter propensity score-matched study (FAVICOV)",
"author": "T Siripongboonsitti",
"doi-asserted-by": "crossref",
"first-page": "14884",
"issue": "1",
"journal-title": "Sci Rep",
"key": "pone.0324903.ref017",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.3390/antibiotics11060805",
"article-title": "Real-World Effectiveness and Optimal Dosage of Favipiravir for Treatment of COVID-19: Results from a Multicenter Observational Study in Thailand",
"author": "P Rattanaumpawan",
"doi-asserted-by": "crossref",
"first-page": "805",
"issue": "6",
"journal-title": "Antibiotics (Basel)",
"key": "pone.0324903.ref018",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.lansea.2023.100166",
"article-title": "Effectiveness of favipiravir monotherapy in the treatment of COVID-19: real world data analysis from Thailand",
"author": "A Srisubat",
"doi-asserted-by": "crossref",
"first-page": "100166",
"journal-title": "Lancet Reg Health Southeast Asia",
"key": "pone.0324903.ref019",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1093/ije/dyaa057",
"article-title": "Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data",
"author": "C Maringe",
"doi-asserted-by": "crossref",
"first-page": "1719",
"issue": "5",
"journal-title": "Int J Epidemiol",
"key": "pone.0324903.ref020",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1016/j.jclinepi.2016.04.014",
"article-title": "Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses",
"author": "M Hernán",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "J Clin Epidemiol",
"key": "pone.0324903.ref021",
"volume": "79",
"year": "2016"
},
{
"key": "pone.0324903.ref022",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: Building an international community of software platform partners",
"author": "PA Harris",
"doi-asserted-by": "crossref",
"first-page": "103208",
"journal-title": "J Biomed Inform",
"key": "pone.0324903.ref023",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"article-title": "Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support",
"author": "PA Harris",
"doi-asserted-by": "crossref",
"first-page": "377",
"issue": "2",
"journal-title": "J Biomed Inform",
"key": "pone.0324903.ref024",
"volume": "42",
"year": "2009"
},
{
"author": "Organization WH",
"key": "pone.0324903.ref025",
"volume-title": "Clinical management of COVID-19: living guideline, 18 August 2023",
"year": "2023"
},
{
"DOI": "10.18637/jss.v074.i07",
"article-title": "Imputation with the R Package VIM",
"author": "A Kowarik",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "J Stat Soft",
"key": "pone.0324903.ref026",
"volume": "74",
"year": "2016"
},
{
"DOI": "10.1093/aje/kwq439",
"article-title": "Doubly robust estimation of causal effects",
"author": "MJ Funk",
"doi-asserted-by": "crossref",
"first-page": "761",
"issue": "7",
"journal-title": "Am J Epidemiol",
"key": "pone.0324903.ref027",
"volume": "173",
"year": "2011"
},
{
"DOI": "10.1001/jamacardio.2017.2922",
"article-title": "Restricted Mean Survival Time as a Measure to Interpret Clinical Trial Results",
"author": "DH Kim",
"doi-asserted-by": "crossref",
"first-page": "1179",
"issue": "11",
"journal-title": "JAMA Cardiol",
"key": "pone.0324903.ref028",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1016/j.jiph.2023.06.011",
"article-title": "When antivirals backfire: An evaluation of favipiravir’s clinical outcomes in critically ill patients with COVID-19: A multicenter cohort study",
"author": "K Al Sulaiman",
"doi-asserted-by": "crossref",
"first-page": "1492",
"issue": "9",
"journal-title": "J Infect Public Health",
"key": "pone.0324903.ref029",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.2147/IJGM.S457198",
"article-title": "Remdesivir versus Favipiravir in Hospitalized Patients with Moderate to Severe COVID-19 Pneumonia: A Propensity Score-Matched Retrospective Cohort Study",
"author": "K Chavalertsakul",
"doi-asserted-by": "crossref",
"first-page": "2163",
"journal-title": "Int J Gen Med",
"key": "pone.0324903.ref030",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.1016/j.jiph.2022.01.013",
"article-title": "Overview of clinical outcome and therapeutic effectiveness of favipiravir in patients with COVID-19 admitted to intensive care unit, Riyadh, Saudi Arabia",
"author": "A Mutair",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "4",
"journal-title": "J Infect Public Health",
"key": "pone.0324903.ref031",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0330",
"article-title": "Recruitment and Results Reporting of COVID-19 Randomized Clinical Trials Registered in the First 100 Days of the Pandemic",
"author": "P Janiaud",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "pone.0324903.ref032",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00412-X",
"article-title": "Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care",
"author": "PL Shah",
"doi-asserted-by": "crossref",
"first-page": "415",
"issue": "5",
"journal-title": "Lancet Respir Med",
"key": "pone.0324903.ref033",
"volume": "11",
"year": "2023"
},
{
"article-title": "Favipiravir in adults with moderate to severe COVID-19: A phase 3 multicentre, randomized, double-blinded, placebo-controlled trial",
"author": "S Shenoy",
"journal-title": "medRxiv",
"key": "pone.0324903.ref034",
"year": "2021"
},
{
"DOI": "10.4103/jfmpc.jfmpc_1058_22",
"article-title": "Evaluation of the effect of favipiravir on patients with COVID-19",
"author": "A Kamali",
"doi-asserted-by": "crossref",
"first-page": "242",
"issue": "2",
"journal-title": "J Family Med Prim Care",
"key": "pone.0324903.ref035",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1038/s41598-024-62302-w",
"article-title": "In-hospital survival of critically ill COVID-19 patients treated with glucocorticoids: a multicenter real-world data study",
"author": "S Angermair",
"doi-asserted-by": "crossref",
"first-page": "12138",
"issue": "1",
"journal-title": "Sci Rep",
"key": "pone.0324903.ref036",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1001/jamanetworkopen.2023.8516",
"article-title": "Dexamethasone for Inpatients With COVID-19 in a National Cohort",
"author": "A Mourad",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "pone.0324903.ref037",
"volume": "6",
"year": "2023"
},
{
"article-title": "Structural Basis of SARS-CoV-2 Polymerase Inhibition by Favipiravir",
"author": "Q Peng",
"first-page": "100080",
"issue": "1",
"journal-title": "Innovation (Camb)",
"key": "pone.0324903.ref038",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S332458",
"article-title": "Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review",
"author": "WF Qomara",
"doi-asserted-by": "crossref",
"first-page": "8557",
"journal-title": "Int J Gen Med",
"key": "pone.0324903.ref039",
"volume": "14",
"year": "2021"
},
{
"article-title": "Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form",
"author": "P Tabarsi",
"first-page": "1",
"issue": "4",
"journal-title": "Iran J Pharm Res",
"key": "pone.0324903.ref040",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2024.106248",
"article-title": "Favipiravir for COVID-19 in adults in the community in PRINCIPLE, an open-label, randomised, controlled, adaptive platform trial of short- and longer-term outcomes",
"author": "FR Hobbs",
"doi-asserted-by": "crossref",
"first-page": "106248",
"issue": "4",
"journal-title": "J Infect",
"key": "pone.0324903.ref041",
"volume": "89",
"year": "2024"
},
{
"DOI": "10.3389/fmed.2022.826247",
"article-title": "Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia",
"author": "S Al-Muhsen",
"doi-asserted-by": "crossref",
"first-page": "826247",
"journal-title": "Front Med (Lausanne)",
"key": "pone.0324903.ref042",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.1060840",
"article-title": "Viral load and its relationship with the inflammatory response and clinical outcomes in hospitalization of patients with COVID-19",
"author": "M Kuri-Ayache",
"doi-asserted-by": "crossref",
"first-page": "1060840",
"journal-title": "Front Immunol",
"key": "pone.0324903.ref043",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19",
"author": "JB Moore",
"doi-asserted-by": "crossref",
"first-page": "473",
"issue": "6490",
"journal-title": "Science",
"key": "pone.0324903.ref044",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.steroids.2023.109348",
"article-title": "Molecular mechanisms of dexamethasone actions in COVID-19: Ion channels and airway surface liquid dynamics",
"author": "BJ Harvey",
"doi-asserted-by": "crossref",
"first-page": "109348",
"journal-title": "Steroids",
"key": "pone.0324903.ref045",
"volume": "202",
"year": "2024"
},
{
"DOI": "10.1038/s41467-024-49756-2",
"article-title": "Distinct pulmonary and systemic effects of dexamethasone in severe COVID-19",
"author": "LPA Neyton",
"doi-asserted-by": "crossref",
"first-page": "5483",
"issue": "1",
"journal-title": "Nat Commun",
"key": "pone.0324903.ref046",
"volume": "15",
"year": "2024"
},
{
"author": "nation T",
"key": "pone.0324903.ref047",
"volume-title": "Locally made Favipiravir tablets used for Covid treatment get FDA approval",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.683296",
"article-title": "Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial",
"author": "C Chen",
"doi-asserted-by": "crossref",
"first-page": "683296",
"journal-title": "Front Pharmacol",
"key": "pone.0324903.ref048",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"article-title": "Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial",
"author": "Y Lou",
"doi-asserted-by": "crossref",
"first-page": "105631",
"journal-title": "Eur J Pharm Sci",
"key": "pone.0324903.ref049",
"volume": "157",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.107522",
"article-title": "Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia",
"author": "M Solaymani-Dodaran",
"doi-asserted-by": "crossref",
"first-page": "107522",
"journal-title": "Int Immunopharmacol",
"key": "pone.0324903.ref050",
"volume": "95",
"year": "2021"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0324903"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "In-hospital mortality outcomes of favipiravir in patients with moderate to severe COVID-19 infection: An emulated target trial using real-world data from the largest field hospital in Thailand",
"type": "journal-article",
"update-policy": "https://doi.org/10.1371/journal.pone.corrections_policy",
"volume": "20"
}