Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(22)00412-X, PIONEER, NCT04373733, Sep 2022 (preprint)
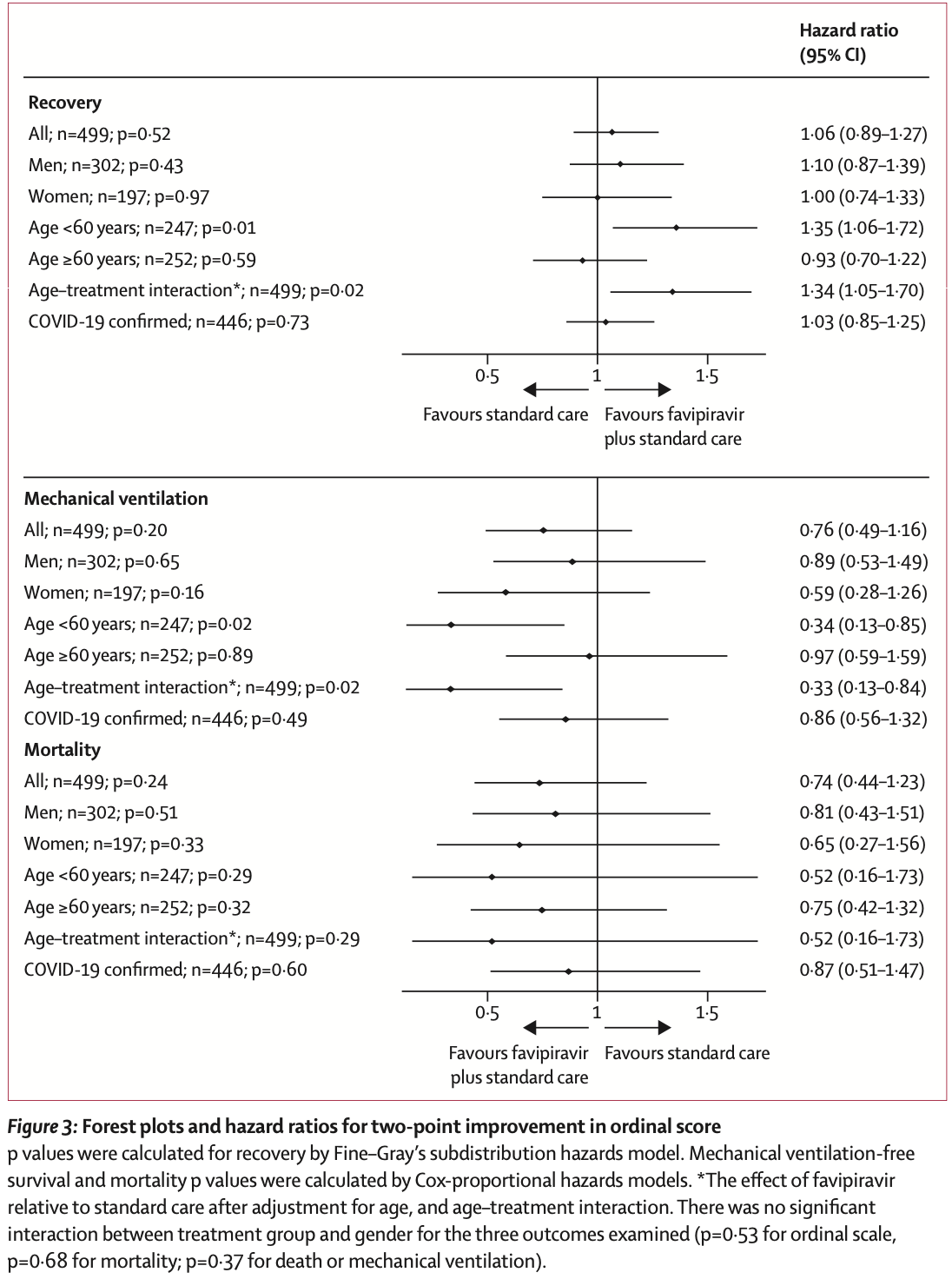
PIONEER very late treatment RCT showing lower mortality and mechanical ventilation with favipiravir, without statistical significance.
The conclusion "favipiravir is not efficacious in treating hospitalised adult patients with COVID-19" is incorrect. Authors show 26% and 24% lower mortality and mechanical ventilation. While these results are not statistically significant, they predict efficacy, and cannot be used to rule out efficacy.
Favipiravir 1,800mg bid day 1, 800mg bid days 2-10.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 26.0% lower, HR 0.74, p = 0.24, treatment 26 of 251 (10.4%), control 34 of 248 (13.7%), NNT 30, day 28.
|
|
risk of mechanical ventilation, 24.0% lower, HR 0.76, p = 0.21, treatment 251, control 248.
|
|
risk of no recovery, 5.7% lower, HR 0.94, p = 0.53, treatment 251, control 248, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Shah et al., 22 Sep 2022, Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, median age 58.9, 120 authors, study period 5 May, 2020 - 26 May, 2021, average treatment delay 8.9 days, trial NCT04373733 (history) (PIONEER).
Contact: pallav.shah@imperial.ac.uk.
Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(22)00412-x
Background COVID-19 has overwhelmed health services globally. Oral antiviral therapies are licensed worldwide, but indications and efficacy rates vary. We aimed to evaluate the safety and efficacy of oral favipiravir in patients hospitalised with COVID-19.
Methods We conducted a multicentre, open-label, randomised controlled trial of oral favipiravir in adult patients who were newly admitted to hospital with proven or suspected COVID-19 across five sites in the UK (n=2), Brazil (n=2) and Mexico (n=1). Using a permuted block design, eligible and consenting participants were randomly assigned (1:1) to receive oral favipiravir (1800 mg twice daily for 1 day; 800 mg twice daily for 9 days) plus standard care, or standard care alone. All caregivers and patients were aware of allocation and those analysing data were aware of the treatment groups. The prespecified primary outcome was the time from randomisation to recovery, censored at 28 days, which was assessed using an intention-to-treat approach. Post-hoc analyses were used to assess the efficacy of favipiravir in patients aged younger than 60 years, and in patients aged 60 years and older. The trial was registered with clinicaltrials.gov, NCT04373733.
Findings Between May 5, 2020 and May 26, 2021, we assessed 503 patients for eligibility, of whom 499 were randomly assigned to favipiravir and standard care (n=251) or standard care alone (n=248). There was no significant difference between those who received favipiravir and standard care, relative to those who received standard care alone in time to recovery in the overall study population (hazard ratio [HR] 1•06 [95% CI 0•89-1•27]; n=499; p=0•52). Post-hoc analyses showed a faster rate of recovery in patients younger than 60 years who received favipiravir and standard care versus those who had standard care alone (HR 1•35 [1•06-1•72]; n=247; p=0•01). 36 serious adverse events were observed in 27 (11%) of 251 patients administered favipiravir and standard care, and 33 events were observed in 27 (11%) of 248 patients receiving standard care alone, with infectious, respiratory, and cardiovascular events being the most numerous. There was no significant between-group difference in serious adverse events per patient (p=0•87). Interpretation Favipiravir does not improve clinical outcomes in all patients admitted to hospital with COVID-19, however, patients younger than 60 years might have a beneficial clinical response. The indiscriminate use of favipiravir globally should be cautioned, and further high-quality studies of antiviral agents, and their potential treatment combinations, are warranted in COVID-19. Funding LifeArc and CW+.
Ziekenhuis, Leuven, Belgium; and Andrew Hill, University of Liverpool, Liverpool, UK) for their oversight. We also thank Paul Cullinan for critical review of the manuscript, Lambert Assoumou for statistical support, and the patients for their altruism in participating in this trial. LifeArc awarded a grant for this study from a £10 million budget that the charity had set aside to fund research into existing or late-stage development therapies that could have the potential to be repurposed for patients with COVID-19. CW+ provided further funding for the study through philanthropic donations from XTX Markets, the Andrew and Belinda Scott Charitable Trust, the Pugh Family, Ageas Insurance, Cadogan, the David and Claudia Harding Foundation, and other private individuals. We are grateful to Fujifilm Toyama Chemical Co for supplying favipiravir, Richard H Kaszynski from Stanford Solutions, Stanford School of Medicine (Standford, CA, USA) for his expert consultation, and to Guy de Selliers for his assistance in the initiation of the study.
References
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of COVID-19-final report, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Ji, Ma, Peppelenbosch, Pan, Potential association between COVID-19 mortality and health-care resource availability, Lancet Glob Health
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Kaptein, Jacobs, Langendries, Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci
Kaptein, Jacobs, Langendries, Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci
Ko, Danielson, Town, Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system, Clin Infect Dis
Mehra, Desai, Ruschitzka, Patel, RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis, Lancet, doi:10.1016/S0140-6736(20)31180-6
Naesens, Guddat, Keough, Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir), Mol Pharmacol
Peckham, De Gruijter, Raine, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission, Nat Commun
Pilkington, Pepperrell, Hill, A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic?, J Virus Erad
Seiglie, Platt, Cromer, Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19, Diabetes Care
Shannon, Selisko, Le, Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis, Nat Commun
Shinkai, Tsushima, Tanaka, Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect Dis Ther
Shiraki, Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol Ther
Sjc, Mughal, Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management, Lancet
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA
Victor, Healy, Thomas, Seargeant, Older patients and delayed discharge from hospital, Health Soc Care Community
Wagner, Saad-Roy, Morris, Vaccine nationalism and the dynamics and control of SARS-CoV-2, Science
Wang, Fan, Horby, Comparative outcomes of adults hospitalized with seasonal influenza A or B virus infection: application of the 7-category ordinal scale. Open forum infectious diseases, Open Forum Infect Dis
Wang, Fan, Salam, Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection, J Infect Dis
DOI record:
{
"DOI": "10.1016/s2213-2600(22)00412-x",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(22)00412-X",
"alternative-id": [
"S221326002200412X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(22)00412-X"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(22)00479-9"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9052-4638",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shah",
"given": "Pallav L",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9167-5462",
"affiliation": [],
"authenticated-orcid": false,
"family": "Orton",
"given": "Christopher M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3692-5155",
"affiliation": [],
"authenticated-orcid": false,
"family": "Grinsztejn",
"given": "Beatriz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5538-4190",
"affiliation": [],
"authenticated-orcid": false,
"family": "Donaldson",
"given": "Gavin C",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2587-1123",
"affiliation": [],
"authenticated-orcid": false,
"family": "Crabtree Ramírez",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tonkin",
"given": "James",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2888-2573",
"affiliation": [],
"authenticated-orcid": false,
"family": "Santos",
"given": "Breno R",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5230-0003",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cardoso",
"given": "Sandra W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ritchie",
"given": "Andrew I",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3429-4759",
"affiliation": [],
"authenticated-orcid": false,
"family": "Conway",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riberio",
"given": "Maria P D",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6398-7450",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wiseman",
"given": "Dexter J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tana",
"given": "Anand",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7794-3435",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vijayakumar",
"given": "Bavithra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caneja",
"given": "Cielito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leaper",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mann",
"given": "Bobby",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samson",
"given": "Anda",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3511-756X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bhavsar",
"given": "Pankaj K",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2213-1103",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boffito",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Mark R",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4091-5323",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pozniak",
"given": "Anton",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelly",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Foster",
"given": "Damon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shabbir",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Connolly",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cartier",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaffer",
"given": "Sajjida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Winpenny",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daby",
"given": "Doris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pepper",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adamson",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carungcong",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nundlall",
"given": "Kribashnie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fedele",
"given": "Serge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samson-Fessale",
"given": "Pardina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schoolmeesters",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes de Almeida Martins",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bull",
"given": "Rhian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Correia Da Costa",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bautista",
"given": "Carina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eleanor Flores",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maheswaran",
"given": "Shameera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Macabodbod",
"given": "Lester",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Houseman",
"given": "Rosalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Svensson",
"given": "Marie-Louise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sayan",
"given": "Amrinder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fung",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garner",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lai",
"given": "Dilys",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nelson",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moore",
"given": "Luke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gidwani",
"given": "Shewta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ouma",
"given": "Beatrice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salinos",
"given": "Clovis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salha",
"given": "Jad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yassein",
"given": "Redasaad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abbasi",
"given": "Abdul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oblak",
"given": "Metod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Steward",
"given": "Angelica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thankachen",
"given": "Mini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barker",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandes",
"given": "Candida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beatriz",
"given": "Veronica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Leah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soler-Carracedo",
"given": "Alfredo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rocca",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maheswaran",
"given": "Shameera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martella",
"given": "Carmela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lloyd",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nolan",
"given": "Ciara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Horsford",
"given": "Latoya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martins",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Lervina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Winstanley",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bourke",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Branch",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orhan",
"given": "Orhan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morton",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunder",
"given": "Sangeetha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patil",
"given": "Shashank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhe",
"given": "Wu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Leon",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farah",
"given": "Ayaan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rya",
"given": "Grace",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alizadeh",
"given": "Katrin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leong",
"given": "Kirsty",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trepte",
"given": "Laure",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goel",
"given": "Nupur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGown",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kirwan",
"given": "Ursula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilela Baião",
"given": "Tamiris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marins",
"given": "Luana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazer",
"given": "Sandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malaguthi de Souza",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feitosa",
"given": "Marcella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lessa",
"given": "Flavia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva de Magalhães",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costenaro",
"given": "Jamile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Cassia Alves Lira",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carolina",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cauduro de Castro",
"given": "Andréa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Machado Da Silva",
"given": "Andre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kliemann",
"given": "Dimas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Cassia Alves Lira",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norton",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lowthorpe",
"given": "Vicki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ivan",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lillie",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Easom",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sierra Madero",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López Iñiguez",
"given": "Álvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patricia Muñuzuri Nájera",
"given": "Guadalupe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paola Alarcón Murra",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alanis Vega",
"given": "Audelia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muñoz Trejo",
"given": "Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez Rodríguez",
"given": "Olivia",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
14
]
],
"date-time": "2022-12-14T23:32:06Z",
"timestamp": 1671060726000
},
"deposited": {
"date-parts": [
[
2022,
12,
14
]
],
"date-time": "2022-12-14T23:32:17Z",
"timestamp": 1671060737000
},
"indexed": {
"date-parts": [
[
2022,
12,
15
]
],
"date-time": "2022-12-15T06:05:42Z",
"timestamp": 1671084342986
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
24
]
],
"date-time": "2022-10-24T00:00:00Z",
"timestamp": 1666569600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S221326002200412X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S221326002200412X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00412-X_bib1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(20)30068-1",
"article-title": "Potential association between COVID-19 mortality and health-care resource availability",
"author": "Ji",
"doi-asserted-by": "crossref",
"first-page": "e480",
"journal-title": "Lancet Glob Health",
"key": "10.1016/S2213-2600(22)00412-X_bib3",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1126/science.abj7364",
"article-title": "Vaccine nationalism and the dynamics and control of SARS-CoV-2",
"author": "Wagner",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/S2213-2600(22)00412-X_bib4",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00412-X_bib5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00412-X_bib6",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00412-X_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/S2213-2600(22)00412-X_bib8",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1016/j.pharmthera.2020.107512",
"article-title": "Favipiravir, an anti-influenza drug against life-threatening RNA virus infections",
"author": "Shiraki",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol Ther",
"key": "10.1016/S2213-2600(22)00412-X_bib9",
"volume": "209",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-18463-z",
"article-title": "Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis",
"author": "Shannon",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S2213-2600(22)00412-X_bib10",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2014441117",
"article-title": "Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity",
"author": "Kaptein",
"doi-asserted-by": "crossref",
"first-page": "26955",
"journal-title": "Proc Natl Acad Sci USA",
"key": "10.1016/S2213-2600(22)00412-X_bib11",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"article-title": "Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial",
"author": "Shinkai",
"doi-asserted-by": "crossref",
"first-page": "2489",
"journal-title": "Infect Dis Ther",
"key": "10.1016/S2213-2600(22)00412-X_bib12",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/S2055-6640(20)30016-9",
"article-title": "A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic?",
"author": "Pilkington",
"doi-asserted-by": "crossref",
"first-page": "45",
"journal-title": "J Virus Erad",
"key": "10.1016/S2213-2600(22)00412-X_bib13",
"volume": "6",
"year": "2020"
},
{
"article-title": "RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis",
"author": "Mehra",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00412-X_bib14",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiz656",
"article-title": "Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1688",
"journal-title": "J Infect Dis",
"key": "10.1016/S2213-2600(22)00412-X_bib15",
"volume": "221",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofz053",
"article-title": "Comparative outcomes of adults hospitalized with seasonal influenza A or B virus infection: application of the 7-category ordinal scale. Open forum infectious diseases",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/S2213-2600(22)00412-X_bib16",
"volume": "6",
"year": "2019"
},
{
"key": "10.1016/S2213-2600(22)00412-X_bib17",
"series-title": "Living guidance for clinical management of COVID-19: living guidance, Nov 23, 2021",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1419",
"article-title": "Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "e695",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2213-2600(22)00412-X_bib18",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00412-X_bib19",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00412-X_bib20",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00412-X_bib21",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00412-X_bib22",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1046/j.1365-2524.2000.00270.x",
"article-title": "Older patients and delayed discharge from hospital",
"author": "Victor",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "Health Soc Care Community",
"key": "10.1016/S2213-2600(22)00412-X_bib23",
"volume": "8",
"year": "2000"
},
{
"DOI": "10.1124/mol.113.087247",
"article-title": "Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir)",
"author": "Naesens",
"doi-asserted-by": "crossref",
"first-page": "615",
"journal-title": "Mol Pharmacol",
"key": "10.1016/S2213-2600(22)00412-X_bib24",
"volume": "84",
"year": "2013"
},
{
"DOI": "10.1073/pnas.2014441117",
"article-title": "Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity",
"author": "Kaptein",
"doi-asserted-by": "crossref",
"first-page": "26955",
"journal-title": "Proc Natl Acad Sci USA",
"key": "10.1016/S2213-2600(22)00412-X_bib25",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)02657-X",
"article-title": "Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management",
"author": "Heskin",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00412-X_bib26",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19741-6",
"article-title": "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission",
"author": "Peckham",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S2213-2600(22)00412-X_bib27",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.2337/dc20-1506",
"article-title": "Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19",
"author": "Seiglie",
"doi-asserted-by": "crossref",
"first-page": "2938",
"journal-title": "Diabetes Care",
"key": "10.1016/S2213-2600(22)00412-X_bib28",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Int J Infect Dis",
"key": "10.1016/S2213-2600(22)00412-X_bib29",
"volume": "102",
"year": "2021"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S221326002200412X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): a multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}