Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form
et al., Iranian Journal of Pharmaceutical Research, doi:10.22037/ijpr.2021.115510.15401, Sep 2021
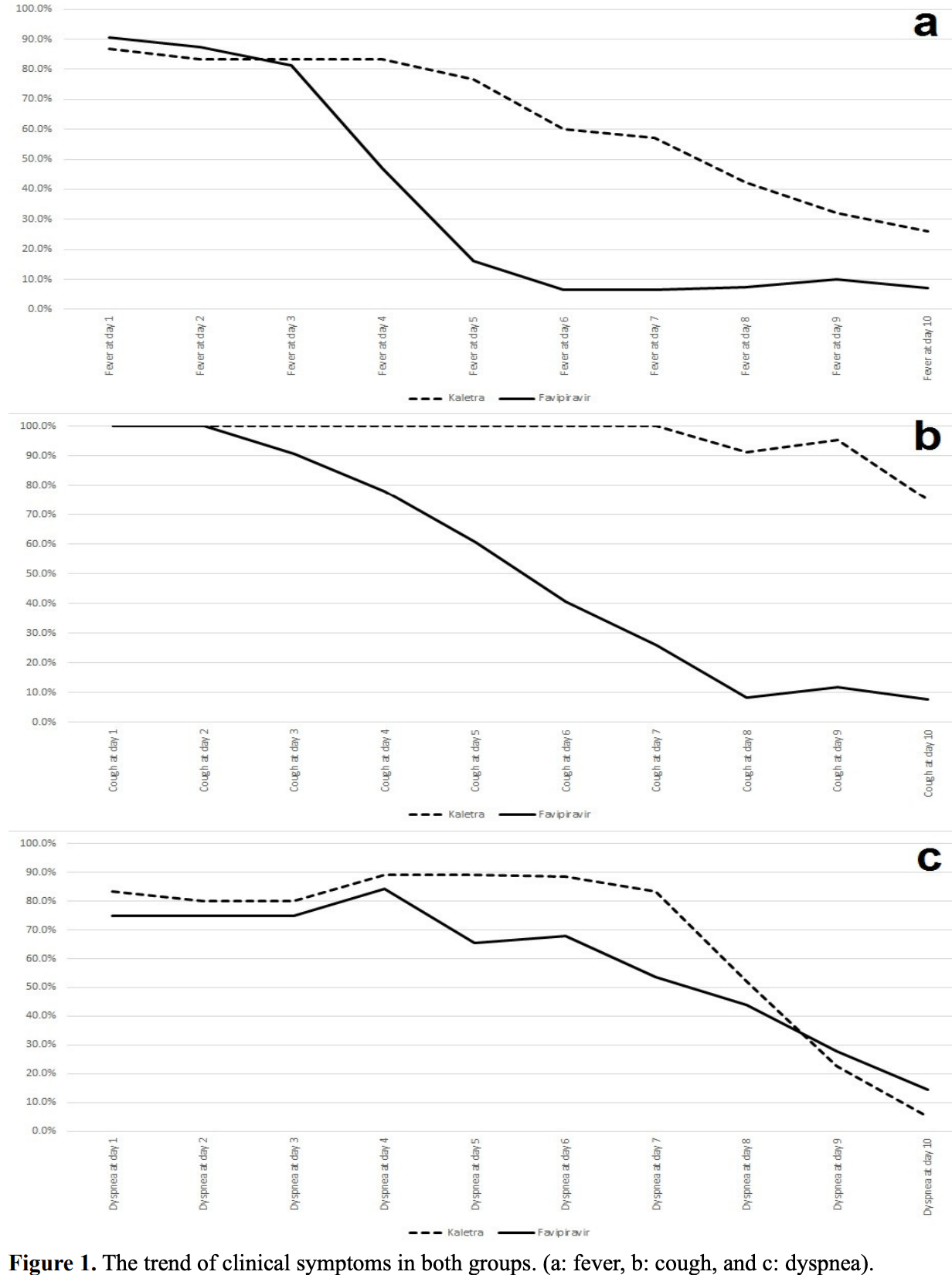
Small 62 patient late stage RCT in Iran comparing favipiravir and lopinavir/ritonavir, showing significant improvement in fever, cough, and dyspnea with favipiravir on day 5. There was no significant difference in mortality, ICU admission, or chest CT improvement. IRCT20151227025726N14.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 29.7% lower, RR 0.70, p = 0.70, treatment 3 of 32 (9.4%), control 4 of 30 (13.3%), NNT 25.
|
|
risk of ICU admission, 41.4% lower, RR 0.59, p = 0.36, treatment 5 of 32 (15.6%), control 8 of 30 (26.7%), NNT 9.1.
|
|
risk of <50% improvement in chest CT, 6.2% lower, RR 0.94, p = 0.76, treatment 24 of 32 (75.0%), control 24 of 30 (80.0%), NNT 20.
|
|
hospitalization time, 25.0% lower, relative time 0.75, p = 0.03, treatment 32, control 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Tabarsi et al., 30 Sep 2021, Randomized Controlled Trial, Iran, peer-reviewed, 27 authors, study period 4 April, 2020 - 7 May, 2020, average treatment delay 7.0 days, this trial compares with another treatment - results may be better when compared to placebo.
Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form
doi:10.22037/ijpr.2021.115510.15401
Coronavirus disease -19 (COVID-19) pandemic, caused by SARS-CoV-2, has gradually spread worldwide, becoming a major public health event. This situation requires designing a novel antiviral agent against the SARS-CoV-2; however, this is time-consuming and the use of repurposed medicines may be promising. One such medicine is favipiravir, primarily introduced as an anti-influenza agent in east world. The aim of this study was to evaluate the efficacy and safety of favipiravir in comparison with lopinavir-ritonavir in SARS-CoV-2 infection. In this randomized clinical trial, 62 patients were recruited. These patients had bilateral pulmonary infiltration with peripheral oxygen saturation lower than 93%. The median time from symptoms onset to intervention initiation was seven days. Favipiravir was not available in the Iranian pharmaceutical market, and it was decided to formulate it at the
References
Boretti, Favipiravir use for SARS CoV-2 infection, Pharmacol. Rep
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering
Coomes, Haghbayan, Favipiravir, an antiviral for COVID-19?, J. Antimicrob. Chemother
Dastan, Nadji, Saffaei, Tabarsi, Tocilizumab administration in a refractory case of COVID-19, Int. J. Antimicrob. Agents
Dastan, Tabarsi, Marjani, Moniri, Hashemian et al., Thalidomide against Coronavirus Disease 2019 (COVID-19): A Medicine with a Thousand Faces, Iran. J. Pharm. Res
Dong, Hu, Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), Drug Discov. Ther
Gioia, Ciaccio, Simone, Fasciglione, Di Masi et al., Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches, Biochem. Pharmacol
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin. Infect. Dis
Jordan, Stevens, Deval, Nucleosides for the treatment of respiratory RNA virus infections, Antivir. Chem. Chemother
Joseph, Dibas, Efficacy and safety of lopinavir/ritonavir in the treatment of COVID-19: A systematic review, Expert Rev. Anti Infect. Ther
Lambert, Petteway, Mcdanal, Hart, Leary et al., Human immunodeficiency virus type 1 protease inhibitors irreversibly block infectivity of purified virions from chronically infected cells, Antimicrob. Agents Chemother
Pan, Ye, Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19), Radiology
Reddy, Lai, Tackling COVID-19 Using Remdesivir and Favipiravir as Therapeutic Options, Chembiochem
Rosa, Santos, Clinical trials on drug repositioning for COVID-19 treatment, Rev. Panam. Salud. Publica
Takian, Raoofi, Kazempour-Ardebili, COVID-19 battle during the toughest sanctions against Iran, Lancet
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int. J. Infect. Dis
Vakili, Fathi, Pezeshgi, Mohamadkhani, Hajiesmaeili et al., Critical complications of COVID-19: A descriptive meta-analysis study, Rev. Cardiovasc. Med
DOI record:
{
"DOI": "10.22037/ijpr.2021.115510.15401",
"URL": "https://doi.org/10.22037/ijpr.2021.115510.15401",
"author": [
{
"family": "Tabarsi",
"given": "Payam"
},
{
"family": "Vahidi",
"given": "Hossein"
},
{
"family": "Saffaei",
"given": "Ali"
},
{
"family": "Hashemian",
"given": "Seyed Mohammad Reza"
},
{
"family": "Jamaati",
"given": "Hamidreza"
},
{
"family": "Daraei",
"given": "Bahram"
},
{
"family": "Mahboubi",
"given": "Arash"
},
{
"family": "Kobarfard",
"given": "Farzad"
},
{
"family": "Marjani",
"given": "Majid"
},
{
"family": "Moniri",
"given": "Afshin"
},
{
"family": "Abtahian",
"given": "Zahra"
},
{
"family": "Abedini",
"given": "Atefeh"
},
{
"family": "Eslaminejad",
"given": "Alireza"
},
{
"family": "Heshmatnia",
"given": "Jalal"
},
{
"family": "Mirenayat",
"given": "Maryam Sadat"
},
{
"family": "Fakharian",
"given": "Atefeh"
},
{
"family": "Seifi",
"given": "Sharareh"
},
{
"family": "Sadeghi",
"given": "Mohsen"
},
{
"family": "Dastan",
"given": "Alireza"
},
{
"family": "Haseli",
"given": "Sara"
},
{
"family": "Nadji",
"given": "Seyed Alireza"
},
{
"family": "Eskandari",
"given": "Raha"
},
{
"family": "Yousefian",
"given": "Sahar"
},
{
"family": "Varahram",
"given": "Mohammad"
},
{
"family": "Zali",
"given": "Alireza"
},
{
"family": "Velayati",
"given": "Ali Akbar"
},
{
"family": "Dastan",
"given": "Farzaneh"
}
],
"container-title": "Iranian Journal of Pharmaceutical Research",
"container-title-short": "IJPR",
"issue": "4",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journalAbbreviation": "IJPR",
"language": "eng",
"publisher": "School of Pharmacy, Shahid Beheshti University of Medical Sciences",
"publisher-place": "IR",
"title": "Favipiravir Effects on the Control of Clinical Symptoms of Hospitalized COVID-19 Cases: An Experience with Iranian Formulated Dosage Form",
"type": "article-journal",
"volume": "20"
}