When Antivirals Backfire: An Evaluation of Favipiravir’s Clinical Outcomes in Critically Ill Patients with COVID-19: A Multicenter Cohort Study
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.06.011, Jun 2023
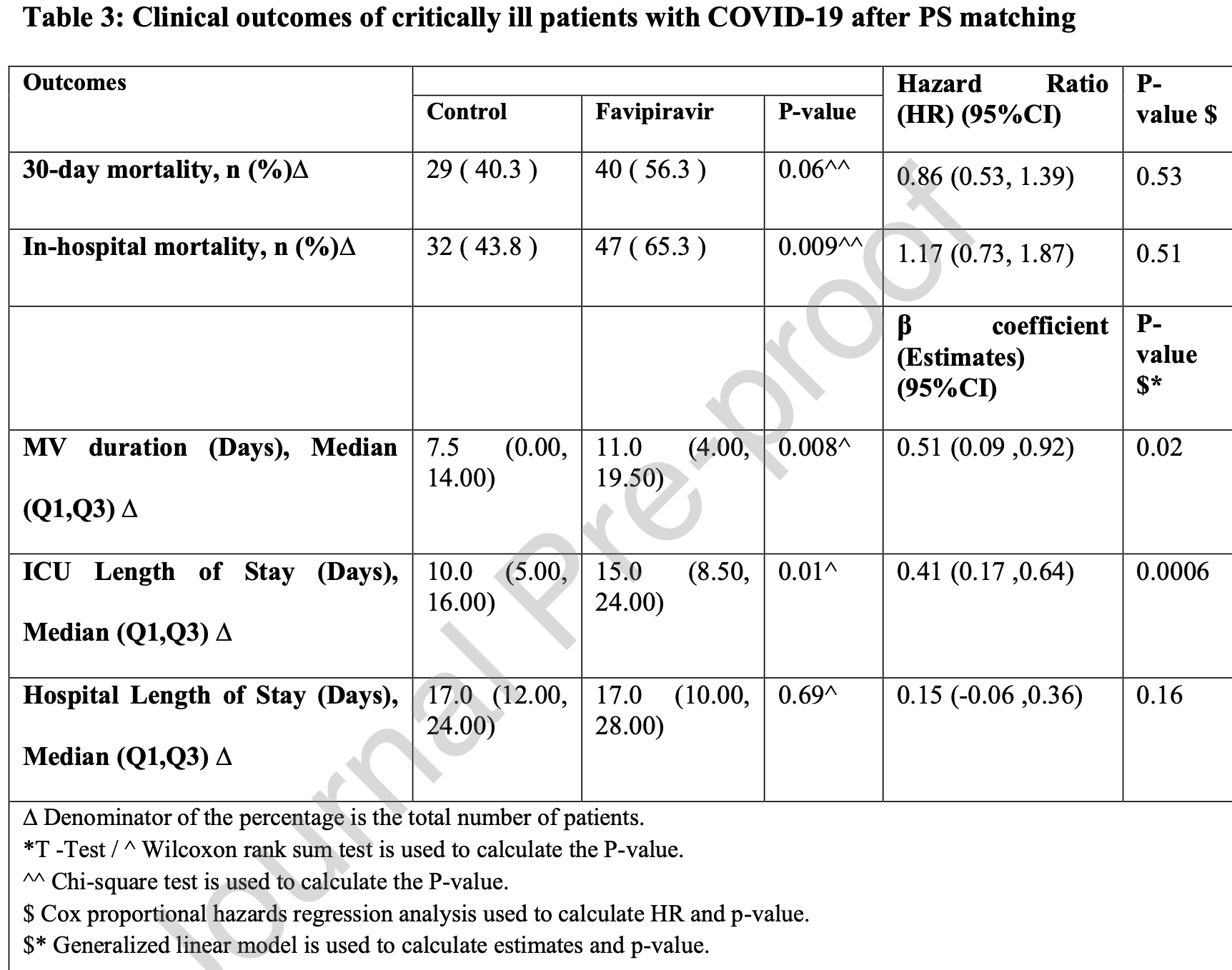
PSM retrospective 1,218 COVID-19 ICU patients in Saudi Arabia, showing no significant difference in mortality, and longer ICU/MV time with favipiravir treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 17.0% higher, HR 1.17, p = 0.51, treatment 73, control 73, in-hospital, propensity score matching.
|
|
risk of death, 14.0% lower, HR 0.86, p = 0.53, treatment 73, control 73, propensity score matching, day 30.
|
|
ventilation time, 46.7% higher, relative time 1.47, p = 0.008, treatment 73, control 73, propensity score matching.
|
|
ICU time, 50.0% higher, relative time 1.50, p = 0.01, treatment 73, control 73, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Sulaiman et al., 14 Jun 2023, retrospective, Saudi Arabia, peer-reviewed, mean age 60.1, 20 authors, study period 1 March, 2020 - 31 July, 2021.
Contact: alsulaimankh@hotmail.com.
When Antivirals Backfire: An Evaluation of Favipiravir’s Clinical Outcomes in Critically Ill Patients with COVID-19: A Multicenter Cohort Study
Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.06.011
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions All authors agreed to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
Ethical Approval and Informed Consent Statement The study protocol was reviewed and approved by the Institutional Review Board of King
J o u r n a l P r e -p r o o f
Conflicts of interest The authors declare no conflict of interest.
Declaration of Competing Interest We have no conflict of interest to declare. J o u r n a l P r e -p r o o f
References
Al-Muhsen, Ns, Sharif-Askari, Basamh, Alyounes et al., Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia, Front Med, doi:10.3389/fmed.2022.826247
Alamer, Alrashed, Alfaifi, Alosaimi, Alhassar et al., Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis, Curr Med Res Opin, doi:10.1080/03007995.2021.1920900
Alhazzani, Alshahrani, Alshamsi, Aljuhani, Eljaaly et al., The Saudi Critical Care Society practice guidelines on the management of COVID-19 in the ICU: Therapy section, J Infect Public Health, doi:10.1016/j.jiph.2021.10.005
Aljuhani, Sulaiman, Alshabasy, Eljaaly, Shaya et al., Association between tocilizumab and emerging multidrug-resistant organisms in critically ill patients with COVID-19: A multicenter, retrospective cohort study, BMC Infect Dis J o u r n a l P r e -p r o o f, doi:10.1186/s12879-021-06813-1
Arabia, Saudi MoH Protocol for Adults Patients Suspected of / Confirmed with COVID-19 Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection
Ford, Vitoria, Rangaraj, Norris, Calmy et al., Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment, J Int AIDS Soc, doi:10.1002/jia2.25489
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Japan Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Furuta, Takahashi, Shiraki, Sakamoto, Smee et al., T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral J, Antiviral Res, doi:10.1016/j.antiviral.2009.02.198
Guner, Hasanoglu, Kayaaslan, Aypak, Akinci et al., Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, favipiravir, and hydroxychloroquine plus favipiravir, J Infect Public Health, doi:10.1016/j.jiph.2020.12.017
Harbi, Kensara, Aljuhani, Korayem, Altebainawi et al., Statins and Risk of Thrombosis in Critically ill Patients with COVID-19: A Multicenter Cohort Study, Clin Appl Thromb, doi:10.1177/10760296221103864
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control, J Clin Med, doi:10.3390/jcm9041225
Irie, Nakagawa, Fujita, Tamura, Eto et al., Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol, doi:10.1002/psp4.12685
Jang, Jeon, Kim, Sy, Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2024302118
Lan, Lai, Chang, Lu, Hung et al., Favipiravir-based treatment for outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, Expert Rev Clin Pharmacol, doi:10.1080/17512433.2022.2078701
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Munir, Kuganda, Basry, The efficacy and safety of antivirus drugs for COVID-19: A systematic review, Syst Rev Pharm, doi:10.31838/srp.2020.7.26
Qomara, Primanissa, Amalia, Purwadi, Zakiyah, Effectiveness of remdesivir, lopinavir/ritonavir, and favipiravir for COVID-19 treatment: A systematic review, Int J Gen Med, doi:10.2147/IJGM.S332458
Scape, Saudi, None, Critical Care Pharmacy Research
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107522
Sulaiman, Aljuhani, Eljaaly, Alharbi, Shabasy et al., Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): A multicenter cohort study, Int J Infect Dis, doi:10.1016/j.ijid.2021.02.037
Sulaiman, Aljuhani, Korayem, Hafiz, Alalawi et al., Standard dosing of enoxaparin versus unfractionated heparin in critically ill patient with COVID-19: a multicenter propensity-score matched study, Thromb J, doi:10.1186/s12959-022-00432-9
Sulaiman, Aljuhani, Shaya, Kharbosh, Kensara et al., Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study, Crit Care, doi:10.1186/s13054-021-03785-1
Sulaiman, Korayem, Altebainawi, Harbi, Alharthi, Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study, Crit Care, doi:10.1186/s13054-022-04158-y
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Paulson, Pease, Watson, Comfort et al., Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, Lancet, doi:10.1016/S0140-6736(21)02796-3
Wang, Zhong, Salam, Tarning, Zhan et al., Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza, J o u r n a l P r e -p r o o f Total Bilirubin, doi:10.1016/j.ebiom.2020.103125
Yang, Zeng, Dai, Chen, Yang, A systematic review and Bayesian network meta-analysis for comparative safety assessment of favipiravir interventions in hospitalized COVID-19 patients, J Infect Dev Ctries, doi:10.3855/jidc.16083
Özlüşen, Kozan, Akcan, Kalender, Yaprak et al., Effectiveness of J o u r n a l P r e -p r o o f favipiravir in COVID-19: a live systematic review, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-021-04307-1
DOI record:
{
"DOI": "10.1016/j.jiph.2023.06.011",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2023.06.011",
"alternative-id": [
"S1876034123002162"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "When Antivirals Backfire: An Evaluation of Favipiravir’s Clinical Outcomes in Critically Ill Patients with COVID-19: A Multicenter Cohort Study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2023.06.011"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Sulaiman",
"given": "Khalid Al",
"sequence": "first"
},
{
"affiliation": [],
"family": "Aljuhani",
"given": "Ohoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Korayem",
"given": "Ghazwa B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Altebainawi",
"given": "Ali F.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4735-1663",
"affiliation": [],
"authenticated-orcid": false,
"family": "AlFaifi",
"given": "Mashael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1933-0988",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nahari",
"given": "Majed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almagthali",
"given": "Alaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thabit",
"given": "Abrar K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8776-3419",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alhajaji",
"given": "Raghad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alharbi",
"given": "Reham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "kahtani",
"given": "Khawla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alenazi",
"given": "Abeer A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alharbi",
"given": "Aisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alghwainm",
"given": "Munirah M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alotaibi",
"given": "Sara M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alghamdi",
"given": "Yazeed S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alotaibi",
"given": "Samar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alonazi",
"given": "Shaden H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almutairi",
"given": "Jumanah M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vishwakarma",
"given": "Ramesh",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
16
]
],
"date-time": "2023-06-16T00:44:18Z",
"timestamp": 1686876258000
},
"deposited": {
"date-parts": [
[
2023,
6,
16
]
],
"date-time": "2023-06-16T00:44:43Z",
"timestamp": 1686876283000
},
"indexed": {
"date-parts": [
[
2023,
6,
16
]
],
"date-time": "2023-06-16T04:20:11Z",
"timestamp": 1686889211006
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 10,
"start": {
"date-parts": [
[
2023,
6,
11
]
],
"date-time": "2023-06-11T00:00:00Z",
"timestamp": 1686441600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123002162?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123002162?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.3390/jcm9041225",
"article-title": "The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control",
"author": "Helmy",
"doi-asserted-by": "crossref",
"first-page": "1225",
"journal-title": "J Clin Med",
"key": "10.1016/j.jiph.2023.06.011_bib1",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)02796-3",
"article-title": "Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1513",
"journal-title": "Lancet (London, England)",
"key": "10.1016/j.jiph.2023.06.011_bib2",
"volume": "399",
"year": "2022"
},
{
"article-title": "Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment",
"author": "Ford",
"first-page": "23",
"journal-title": "J Int AIDS Soc",
"key": "10.1016/j.jiph.2023.06.011_bib3",
"year": "2020"
},
{
"article-title": "The efficacy and safety of antivirus drugs for COVID-19: A systematic review",
"author": "Munir",
"first-page": "162",
"journal-title": "Syst Rev Pharm",
"key": "10.1016/j.jiph.2023.06.011_bib4",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2024302118",
"article-title": "Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay",
"author": "Jang",
"doi-asserted-by": "crossref",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.jiph.2023.06.011_bib5",
"volume": "118",
"year": "2021"
},
{
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"author": "Manabe",
"first-page": "21",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jiph.2023.06.011_bib6",
"year": "2021"
},
{
"DOI": "10.1007/s10096-021-04307-1",
"article-title": "Effectiveness of favipiravir in COVID-19: a live systematic review",
"author": "Özlüşen",
"doi-asserted-by": "crossref",
"first-page": "2575",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "10.1016/j.jiph.2023.06.011_bib7",
"volume": "40",
"year": "2021"
},
{
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"first-page": "11",
"journal-title": "Sci Rep",
"key": "10.1016/j.jiph.2023.06.011_bib8",
"year": "2021"
},
{
"DOI": "10.1080/03007995.2021.1920900",
"article-title": "Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis",
"author": "Alamer",
"doi-asserted-by": "crossref",
"first-page": "1085",
"journal-title": "Curr Med Res Opin",
"key": "10.1016/j.jiph.2023.06.011_bib9",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.jiph.2021.10.005",
"article-title": "The Saudi Critical Care Society practice guidelines on the management of COVID-19 in the ICU: Therapy section",
"author": "Alhazzani",
"doi-asserted-by": "crossref",
"first-page": "142",
"journal-title": "J Infect Public Health",
"key": "10.1016/j.jiph.2023.06.011_bib10",
"volume": "15",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2023.06.011_bib11",
"unstructured": "SCAPE. Saudi Critical Care Pharmacy Research n.d. 〈https://www.scape-platform.com/〉."
},
{
"DOI": "10.1016/j.ijid.2021.02.037",
"article-title": "Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): A multicenter cohort study",
"author": "Al Sulaiman",
"doi-asserted-by": "crossref",
"first-page": "180",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2023.06.011_bib12",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06813-1",
"article-title": "Association between tocilizumab and emerging multidrug-resistant organisms in critically ill patients with COVID-19: A multicenter, retrospective cohort study",
"author": "Aljuhani",
"doi-asserted-by": "crossref",
"first-page": "1127",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jiph.2023.06.011_bib13",
"volume": "21",
"year": "2021"
},
{
"article-title": "Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study",
"author": "Al Sulaiman",
"first-page": "26",
"journal-title": "Crit Care",
"key": "10.1016/j.jiph.2023.06.011_bib14",
"year": "2022"
},
{
"article-title": "Standard dosing of enoxaparin versus unfractionated heparin in critically ill patient with COVID-19: a multicenter propensity-score matched study",
"author": "Al Sulaiman",
"first-page": "20",
"journal-title": "Thromb J",
"key": "10.1016/j.jiph.2023.06.011_bib15",
"year": "2022"
},
{
"article-title": "Statins and Risk of Thrombosis in Critically ill Patients with COVID-19: A Multicenter Cohort Study",
"author": "Al Harbi",
"first-page": "28",
"journal-title": "Clin Appl Thromb",
"key": "10.1016/j.jiph.2023.06.011_bib16",
"year": "2022"
},
{
"article-title": "Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study",
"author": "Al Sulaiman",
"first-page": "25",
"journal-title": "Crit Care",
"key": "10.1016/j.jiph.2023.06.011_bib17",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2022.826247",
"article-title": "Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia",
"author": "Al-Muhsen",
"doi-asserted-by": "crossref",
"first-page": "358",
"journal-title": "Front Med",
"key": "10.1016/j.jiph.2023.06.011_bib18",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1080/17512433.2022.2078701",
"article-title": "Favipiravir-based treatment for outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Lan",
"doi-asserted-by": "crossref",
"journal-title": "Expert Rev Clin Pharmacol",
"key": "10.1016/j.jiph.2023.06.011_bib19",
"year": "2022"
},
{
"DOI": "10.3855/jidc.16083",
"article-title": "A systematic review and Bayesian network meta-analysis for comparative safety assessment of favipiravir interventions in hospitalized COVID-19 patients",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "1406",
"journal-title": "J Infect Dev Ctries",
"key": "10.1016/j.jiph.2023.06.011_bib20",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/j.jiph.2020.12.017",
"article-title": "Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, favipiravir, and hydroxychloroquine plus favipiravir",
"author": "Guner",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "J Infect Public Health",
"key": "10.1016/j.jiph.2023.06.011_bib21",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1002/psp4.12685",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "crossref",
"first-page": "1161",
"journal-title": "CPT Pharmacometrics Syst Pharmacol",
"key": "10.1016/j.jiph.2023.06.011_bib22",
"volume": "10",
"year": "2021"
},
{
"key": "10.1016/j.jiph.2023.06.011_bib23",
"unstructured": "Saudi Arabia MOH. Saudi MoH Protocol for Adults Patients Suspected of / Confirmed with COVID-19 Supportive care and antiviral treatment of suspected or confirmed COVID-19 infection 2022:1–6."
},
{
"article-title": "Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia",
"author": "Solaymani-Dodaran",
"first-page": "95",
"journal-title": "Int Immunopharmacol",
"key": "10.1016/j.jiph.2023.06.011_bib24",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S332458",
"article-title": "Effectiveness of remdesivir, lopinavir/ritonavir, and favipiravir for COVID-19 treatment: A systematic review",
"author": "Qomara",
"doi-asserted-by": "crossref",
"first-page": "8557",
"journal-title": "Int J Gen Med",
"key": "10.1016/j.jiph.2023.06.011_bib25",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2009.02.198",
"article-title": "T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "95",
"journal-title": "Antiviral Res",
"key": "10.1016/j.jiph.2023.06.011_bib26",
"volume": "82",
"year": "2009"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.jiph.2023.06.011_bib27",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Proc Japan Acad Ser B Phys Biol Sci",
"key": "10.1016/j.jiph.2023.06.011_bib28",
"volume": "93",
"year": "2017"
},
{
"article-title": "Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza",
"author": "Wang",
"first-page": "62",
"journal-title": "EBioMedicine",
"key": "10.1016/j.jiph.2023.06.011_bib29",
"year": "2020"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034123002162"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "When Antivirals Backfire: An Evaluation of Favipiravir’s Clinical Outcomes in Critically Ill Patients with COVID-19: A Multicenter Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}