Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.826247, Mar 2022
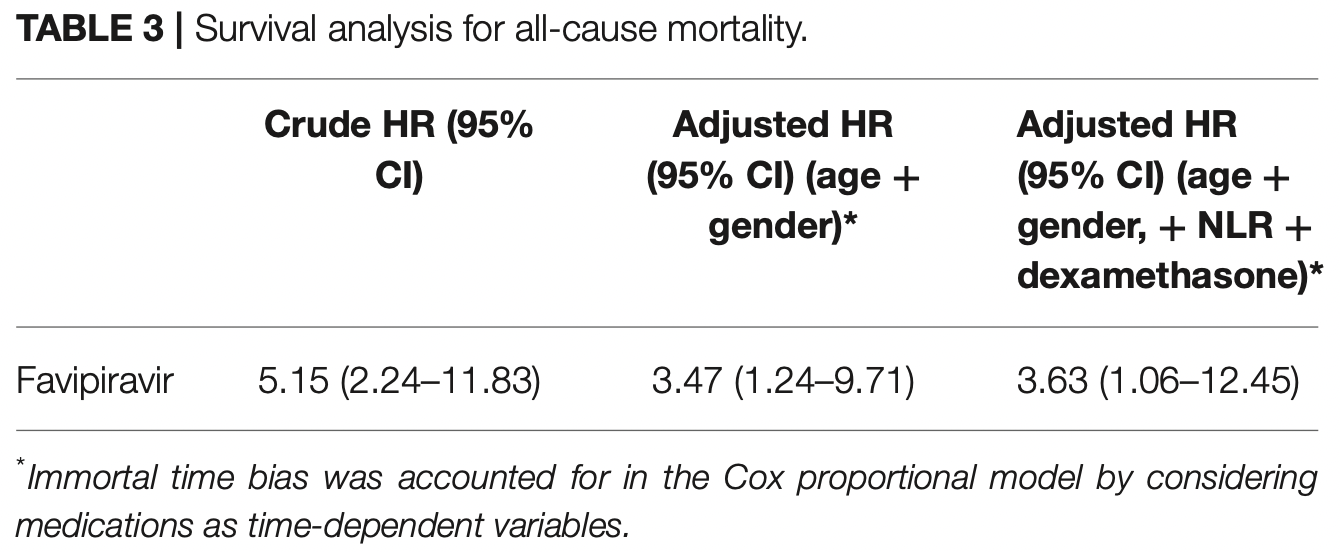
Prospective observational study of 598 hospitalized patients in Saudi Arabia, showing higher risk of mortality and longer hospitalization time with favipiravir.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 263.0% higher, HR 3.63, p = 0.04, treatment 156, control 442, Cox proportional hazards, day 65.
|
|
risk of oxygen therapy, 40.6% lower, RR 0.59, p < 0.001, treatment 52 of 156 (33.3%), control 248 of 442 (56.1%), NNT 4.4.
|
|
hospitalization time, 40.0% higher, relative time 1.40, p = 0.03, treatment 156, control 442.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Al-Muhsen et al., 4 Mar 2022, prospective, Saudi Arabia, peer-reviewed, 11 authors, study period June 2020 - January 2021.
Contact: almuhsen@ksu.edu.sa, rhalwani@sharjah.ac.ae, hayakbaa@gmail.com.
Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia
Frontiers in Medicine, doi:10.3389/fmed.2022.826247
Objectives: There are limited data on the efficacy and safety of favipiravir antiviral in coronavirus disease 2019 , particularly in the more progressed disease phase. This study aims to evaluate the favipiravir effect on reducing the length of hospital stay and in-hospital mortality among moderate and severe hospitalized COVID-19 patients. Methods: A prospective, multicenter observational study was conducted that included moderate and severe hospitalized adult COVID-19 patients in four major regions (Riyadh (Riyadh), Eastern (Dammam), Al-Qassem (Buraydah), and Macca (Jeddah) of Saudi Arabia. For the primary outcome of all-cause mortality, a Cox proportional hazard analysis was performed. While the association between favipiravir use and length of hospital stay was determined using adjusted generalized linear model. This study was approved by the Central Institutional Review Board in The Saudi Ministry of Health (MoH) with the approval number IRB # 20-85-M. Results: This study included 598 moderate and severe COVID-19 patients, of whom 156 (26%) received favipiravir. Favipiravir treatment was associated with more extended hospital stays (14 vs. 10 median days, P = 0.034) and higher mortality rate (aHR 3.63; 95% CI 1.06-12.45) compared to no favipiravir regimen. Despite lack of effectiveness, favipiravir use was only associated with higher diarrhea adverse effects (12 vs. 5%, P = 0.002), but it did not affect the renal and liver profiles of patients.
Conclusion: Favipiravir was ineffective in reducing the length of hospital stay and in-hospital mortality in patients with moderate and severe COVID-19.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by Central Institutional Review Board in The Saudi Ministry of Health (MoH) approval number (IRB # 20-85-M). The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS HA-S, RH, SA-M, NA-N, MA, FA, and NS conceived and designed the study. RB, BA, and AJ collected the data. NS, FS, and RH analyzed the data. All authors contributed to writing and revision of the manuscript. Conflict of Interest: HA-S was employed by Hevolution Foundation.
FUNDING The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alamer, Alrashed, Alfaifi, Alosaimi, Alhassar et al., Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis, Curr Med Res Opin, doi:10.1080/03007995.2021.1920900
Bosaeed, Alharbi, Altayib, Albayat, Alharbi, Favipiravir and hydroxychloroquine combination therapy in patients with moderate to severe COVID-19 (FACCT Trial): an open-label, multicenter, randomized, controlled trial, Infect Dis Therapy, doi:10.2139/ssrn.3829663
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Driouich, Cochin, Lingas, Moureau, Touret et al., Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun, doi:10.1038/s41467-021-21992-w
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep, doi:10.1038/s41598-021-90551-6
Hussain Alsayed, Sharif-Askari, Sharif-Askari, Hussain, Hamid et al., Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: a retrospective cohort study, PLoS ONE, doi:10.1371/journal.pone.0258643
Irie, Nakagawa, Fujita, Tamura, Eto et al., Population pharmacokinetics of favipiravir in patients with COVID-19, CPT Pharmacometrics Syst Pharmacol, doi:10.1111/cts.12827
Jang, Jeon, Kim, Sy, Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay, Proc Nat Acad Sci, doi:10.1073/pnas.2024302118
Lan, Xu, Ye, Xia, Wang et al., Positive RT-PCR test results in patients recovered from COVID-19, JAMA, doi:10.1001/jama.2020.2783
Malhani, Enani, Sharif-Askari, Alghareeb, Bin-Brikan et al., Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: a cohort study, PLoS ONE, doi:10.1371/journal.pone.0252984
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Nagata, Lefor, Hasegawa, Ishii, Favipiravir: a new medication for the Ebola virus disease pandemic, Disaster Med Public Health Prep, doi:10.1017/dmp.2014.151
Rosenke, Feldmann, Westover, Hanley, Martellaro et al., Use of favipiravir to treat lassa virus infection in macaques, Emerg Infect Dis, doi:10.3201/eid2409.180233
Tleyjeh, Ghomrawi, Steckelberg, Montori, Hoskin et al., Conclusion about the association between valve surgery and mortality in an infective endocarditis cohort changed after adjusting for survivor bias, J Clin Epidemiol, doi:10.1016/j.jclinepi.2008.06.022
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, openlabel, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Ueda, Tanimoto, Murayama, Ozaki, Kami, Japan's drug regulation during the COVID-19 pandemic: lessons from a case study of favipiravir, Clin Pharmacol Ther, doi:10.1002/cpt.2251
Wang, Fu, Huang, Han, Zhang et al., Neutrophil-to-lymphocyte ratio on admission is an independent risk factor for the severity and mortality in patients with coronavirus disease 2019, J Infect, doi:10.1016/j.jinf.2020.09.022
Yan, Li, Wang, Yan, Zhu et al., Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study, J Med Virol, doi:10.1002/jmv.26061~
Özlüşen, Kozan, Akcan, Kalender, Yaprak et al., Effectiveness of favipiravir in COVID-19: a live systematic review, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-021-04307-1
DOI record:
{
"DOI": "10.3389/fmed.2022.826247",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.826247",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>There are limited data on the efficacy and safety of favipiravir antiviral in coronavirus disease 2019 (COVID-19), particularly in the more progressed disease phase. This study aims to evaluate the favipiravir effect on reducing the length of hospital stay and in-hospital mortality among moderate and severe hospitalized COVID-19 patients.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A prospective, multicenter observational study was conducted that included moderate and severe hospitalized adult COVID-19 patients in four major regions (Riyadh (Riyadh), Eastern (Dammam), Al-Qassem (Buraydah), and Macca (Jeddah) of Saudi Arabia. For the primary outcome of all-cause mortality, a Cox proportional hazard analysis was performed. While the association between favipiravir use and length of hospital stay was determined using adjusted generalized linear model. This study was approved by the Central Institutional Review Board in The Saudi Ministry of Health (MoH) with the approval number IRB # 20-85-M.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>This study included 598 moderate and severe COVID-19 patients, of whom 156 (26%) received favipiravir. Favipiravir treatment was associated with more extended hospital stays (14 vs. 10 median days, <jats:italic>P</jats:italic> = 0.034) and higher mortality rate (aHR 3.63; 95% CI 1.06–12.45) compared to no favipiravir regimen. Despite lack of effectiveness, favipiravir use was only associated with higher diarrhea adverse effects (12 vs. 5%, <jats:italic>P</jats:italic> = 0.002), but it did not affect the renal and liver profiles of patients.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Favipiravir was ineffective in reducing the length of hospital stay and in-hospital mortality in patients with moderate and severe COVID-19.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.826247"
],
"author": [
{
"affiliation": [],
"family": "Al-Muhsen",
"given": "Saleh",
"sequence": "first"
},
{
"affiliation": [],
"family": "Al-Numair",
"given": "Nouf S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saheb Sharif-Askari",
"given": "Narjes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basamh",
"given": "Roaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alyounes",
"given": "Banan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jabaan",
"given": "Amjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saheb Sharif-Askari",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alosaimi",
"given": "Mohammed F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsohime",
"given": "Fahad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halwani",
"given": "Rabih",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Saud",
"given": "Haya",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
4
]
],
"date-time": "2022-03-04T09:52:22Z",
"timestamp": 1646387542000
},
"deposited": {
"date-parts": [
[
2022,
3,
4
]
],
"date-time": "2022-03-04T09:52:23Z",
"timestamp": 1646387543000
},
"indexed": {
"date-parts": [
[
2022,
4,
3
]
],
"date-time": "2022-04-03T15:33:59Z",
"timestamp": 1649000039744
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
3,
4
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
4
]
],
"date-time": "2022-03-04T00:00:00Z",
"timestamp": 1646352000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.826247/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
3,
4
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
4
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1073/pnas.2024302118",
"article-title": "Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay",
"author": "Jang",
"doi-asserted-by": "publisher",
"first-page": "e2024302118",
"journal-title": "Proc Nat Acad Sci USA.",
"key": "B1",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci.",
"key": "B2",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1017/dmp.2014.151",
"article-title": "Favipiravir: a new medication for the Ebola virus disease pandemic",
"author": "Nagata",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Disaster Med Public Health Prep.",
"key": "B3",
"volume": "9",
"year": "2015"
},
{
"DOI": "10.3201/eid2409.180233",
"article-title": "Use of favipiravir to treat lassa virus infection in macaques",
"author": "Rosenke",
"doi-asserted-by": "publisher",
"first-page": "1696",
"journal-title": "Emerg Infect Dis.",
"key": "B4",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1002/cpt.2251",
"article-title": "Japan's drug regulation during the COVID-19 pandemic: lessons from a case study of favipiravir",
"author": "Ueda",
"doi-asserted-by": "publisher",
"first-page": "545",
"journal-title": "Clin Pharmacol Ther",
"key": "B5",
"volume": "113",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"author": "Manabe",
"doi-asserted-by": "publisher",
"first-page": "489",
"journal-title": "BMC Infect Dis.",
"key": "B6",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.2139/ssrn.3829663",
"article-title": "Favipiravir and hydroxychloroquine combination therapy in patients with moderate to severe COVID-19 (FACCT Trial): an open-label, multicenter, randomized, controlled trial",
"author": "Bosaeed",
"doi-asserted-by": "publisher",
"first-page": "2291",
"journal-title": "Infect Dis Therapy.",
"key": "B7",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0252984",
"article-title": "Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: a cohort study",
"author": "A. Malhani",
"doi-asserted-by": "publisher",
"first-page": "e0252984",
"journal-title": "PLoS ONE",
"key": "B8",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1007/s10096-021-04307-1",
"article-title": "Effectiveness of favipiravir in COVID-19: a live systematic review",
"author": "Özlüşen",
"doi-asserted-by": "publisher",
"first-page": "2575",
"journal-title": "Eur J Clin Microbiol Infect Dis.",
"key": "B9",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1080/03007995.2021.1920900",
"article-title": "Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis",
"author": "Alamer",
"doi-asserted-by": "publisher",
"first-page": "1085",
"journal-title": "Curr Med Res Opin.",
"key": "B10",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "publisher",
"first-page": "11022",
"journal-title": "Sci Rep.",
"key": "B11",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinepi.2008.06.022",
"article-title": "Conclusion about the association between valve surgery and mortality in an infective endocarditis cohort changed after adjusting for survivor bias",
"author": "Tleyjeh",
"doi-asserted-by": "publisher",
"first-page": "130",
"journal-title": "J Clin Epidemiol.",
"key": "B12",
"volume": "63",
"year": "2010"
},
{
"DOI": "10.1371/journal.pone.0258643",
"article-title": "Early administration of remdesivir to COVID-19 patients associates with higher recovery rate and lower need for ICU admission: a retrospective cohort study",
"author": "Hussain Alsayed",
"doi-asserted-by": "publisher",
"first-page": "e0258643",
"journal-title": "PLoS ONE.",
"key": "B13",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"doi-asserted-by": "publisher",
"first-page": "1192",
"journal-title": "Engineering.",
"key": "B14",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int J Infect Dis.",
"key": "B15",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.2783",
"article-title": "Positive RT-PCR test results in patients recovered from COVID-19",
"author": "Lan",
"doi-asserted-by": "publisher",
"first-page": "1502",
"journal-title": "JAMA.",
"key": "B16",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"article-title": "Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model",
"author": "Driouich",
"doi-asserted-by": "publisher",
"first-page": "1735",
"journal-title": "Nat Commun.",
"key": "B17",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/cts.12827",
"article-title": "Population pharmacokinetics of favipiravir in patients with COVID-19",
"author": "Irie",
"doi-asserted-by": "publisher",
"first-page": "1161",
"journal-title": "CPT Pharmacometrics Syst Pharmacol.",
"key": "B18",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "B19",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.09.022",
"article-title": "Neutrophil-to-lymphocyte ratio on admission is an independent risk factor for the severity and mortality in patients with coronavirus disease 2019",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "e16",
"journal-title": "J Infect.",
"key": "B20",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26061",
"article-title": "Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study",
"author": "Yan",
"doi-asserted-by": "publisher",
"first-page": "2573",
"journal-title": "J Med Virol.",
"key": "B21",
"volume": "92",
"year": "2020"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.826247/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Favipiravir Effectiveness and Safety in Hospitalized Moderate-Severe COVID-19 Patients: Observational Prospective Multicenter Investigation in Saudi Arabia",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}