Evaluation of Zinc Sulfate as an Adjunctive Therapy in COVID-19 Critically Ill Patients: a Two Center Propensity-score Matched Study
et al., Critical Care, doi:10.1186/s13054-021-03785-1, Jun 2021 (preprint)
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000028 from 47 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
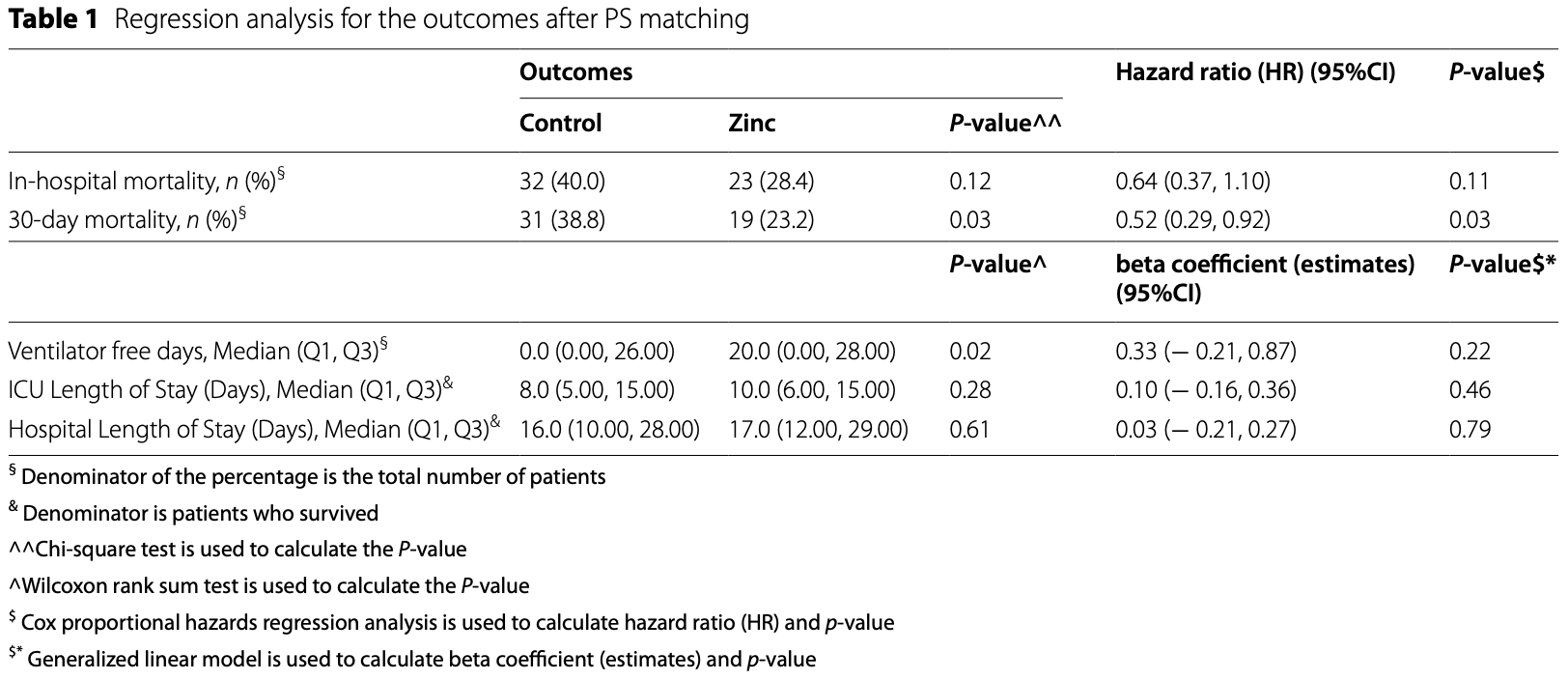
Retrospective 266 ICU patients showing lower mortality with zinc treatment, reaching statistical significance only for 30 day mortality, and lower odds of acute kidney injury, without statistical significance. NRC21R/287/07.
|
risk of death, 36.0% lower, HR 0.64, p = 0.11, treatment 23 of 82 (28.0%), control 32 of 82 (39.0%), NNT 9.1, adjusted per study, in-hospital, PSM, multivariable Cox proportional hazards.
|
|
risk of death, 48.0% lower, HR 0.52, p = 0.03, treatment 19 of 82 (23.2%), control 31 of 82 (37.8%), NNT 6.8, adjusted per study, 30 day, PSM, multivariable Cox proportional hazards.
|
|
ICU time, 25.0% higher, relative time 1.25, p = 0.28, treatment 82, control 82.
|
|
hospitalization time, 6.2% higher, relative time 1.06, p = 0.61, treatment 82, control 82.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Al Sulaiman et al., 7 Jun 2021, retrospective, propensity score matching, Saudi Arabia, peer-reviewed, 11 authors, study period 1 March, 2020 - 31 March, 2021.
Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study
Critical Care, doi:10.1186/s13054-021-03785-1
Background: Zinc is a trace element that plays a role in stimulating innate and acquired immunity. The role of zinc in critically ill patients with COVID-19 remains unclear. This study aims to evaluate the efficacy and safety of zinc sulfate as adjunctive therapy in critically ill patients with COVID-19. Methods: Patients aged ≥ 18 years with COVID-19 who were admitted to the intensive care unit (ICU) in two tertiary hospitals in Saudi Arabia were retrospectively assessed for zinc use from March 1, 2020 until March 31, 2021. After propensity score matching (1:1 ratio) based on the selected criteria, we assessed the association of zinc used as adjunctive therapy with the 30-day mortality. Secondary outcomes included the in-hospital mortality, ventilator free days, ICU length of stay (LOS), hospital LOS, and complication (s) during ICU stay. Results: A total of 164 patients were included, 82 patients received zinc. Patients who received zinc sulfate as adjunctive therapy have a lower 30-day mortality (HR 0.52, CI 0.29, 0.92; p = 0.03). On the other hand, the in-hospital mortality was not statistically significant between the two groups (HR 0.64, CI 0.37-1.10; p = 0.11). Zinc sulfate use was associated with a lower odds of acute kidney injury development during ICU stay (OR 0.46 CI 0.19-1.06; p = 0.07); however, it did not reach statistical significance.
Conclusion: The use of zinc sulfate as an additional treatment in critically ill COVID-19 patients may improve survival. Furthermore, zinc supplementation may have a protective effect on the kidneys.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13054-021-03785-1.
Additional file 1: Table e1 Patients Baseline characteristics before and after propensity-score matching.
Authors' contributions KS and OA equally contributed to the conception and design of the research; AS, GK, AK, SA, RK, RV and AA contributed to the design of the research; KS, OA, AS, GK, AK, SA, RK, RV, AAB, RG and AA contributed to the acquisition and analysis of the data; KS, OA, AS, GK contributed to the interpretation of the data; KS, OA, AS, SA, AK, RK, AA, AAB, RG and RV drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Declarations Ethics approval and consent to participate The study was approved in November 19th, 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Reference No: RC20/589/R). Participants' confidentiality was strictly observed throughout the study using the anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center.
Consent for publication Not applicable.
Competing interests The authors declare that they have no competing..
References
Abd-Elsalam, Soliman, Esmail, Khalaf, Mostafa et al., Do zinc supplements enhance the clinical efficacy of hydroxychloroquine? A randomized multicenter trial, Biol Trace Elem Res
Al Sulaiman, Aljuhani, Eljaaly, Alharbi, Shabasy et al., Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study, Int J Infect Dis
Al-Doush, El-Din, The distribution of selenium levels in Saudi dairy farms: a preliminary report from Al-Kharj, J Environ Pathol Toxicol Oncol
Aleissa, Silverman, Acosta, Nutt, Richterman et al., New perspectives on antimicrobial agents: remdesivir treatment for COVID-19, Antimicrob Agents Chemother
Alhazzani, Evans, Alshamsi, Møller, Ostermann et al., Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update, Critical Care Med
Alhazzani, Møller, Arabi, Loeb, Gong, Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med, doi:10.1007/s00134-020-06022-5
Barnard, Wong, Bailey, Day, Sidwell et al., Effect of oral gavage treatment with ZnAL42 and other metallo-ion formulations on influenza A H5N1 and H1N1 virus infections in mice, Antiviral Chem Chemother
Carlucci, Ahuja, Petrilli, Rajagopalan, Jones et al., Hydroxychloroquine and azithromycin plus zinc vs. hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients, J Medical Microbiol
Chen, Zhao, Qu, Chen, Xiong et al., Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients, Clin Infect Dis
Coomes, Haghbayan, Interleukin-6 in COVID-19: a systematic review and meta-analysis, Rev Med Virol
Derwand, Scholz, Zelenko, COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study, Int J Antimicrob Agents
Fromonot, Gette, Lassoued, Guéant, Guéant-Rodriguez et al., Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19, Clin Nutr, doi:10.1016/j.clnu.2021.04.042
Gupta, Wang, Hayek, Chan, Mathews et al., Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19, JAMA Intern Med
Heyland, Jones, Cvijanovich, Wong, Review: zinc supplementation in critically ill patients: a key pharmaconutrient?, J Parenter Enter Nutr
Huang, Pranata, Lim, Oehadian, Alisjahbana, C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis, Ther Adv Respir Dis, doi:10.1177/1753466620937175
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet
Kumar, Kubota, Chernov, Kasuya, Potential role of zinc supplementation in prophylaxis and treatment of COVID-19, Med Hypotheses
Mdcalc, MDcalc-Medical calculators, equations, scores, and guidelines
Perera, El Khoury, Chinni, Bolton, Qu et al., Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol, BMJ Open
Pormohammad, Monych, Turner, Pormohammad, Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNAdependent RNA-polymerase and 3C-like proteinase enzymes, Int J Mol Med
Read, Obeid, Ahlenstiel, Ahlenstiel, The role of zinc in antiviral immunity, Adv Nutr
Shakoor, Feehan, Dhaheri, Ali, Platat et al., Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19?, Maturitas
Short, Gupta, Brenner, Hayek, Srivastava et al., D-dimer and Death in critically ill patients with coronavirus disease 2019, Crit Care Med
Szarpak, Pruc, Gasecka, Jaguszewski, Michalski et al., Should we supplement zinc in COVID-19 patients? Evidence from meta-analysis, Polish Arch Intern Med
The, Group, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Veiga, Prats, Farias, Rosa, Dourado et al., Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial, BMJ
Velthuis, Van Den Worml, Sims, Baric, Snijder et al., Zn 2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, PLoS Pathog
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
DOI record:
{
"DOI": "10.1186/s13054-021-03785-1",
"ISSN": [
"1364-8535"
],
"URL": "http://dx.doi.org/10.1186/s13054-021-03785-1",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Zinc is a trace element that plays a role in stimulating innate and acquired immunity. The role of zinc in critically ill patients with COVID-19 remains unclear. This study aims to evaluate the efficacy and safety of zinc sulfate as adjunctive therapy in critically ill patients with COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Patients aged ≥ 18 years with COVID-19 who were admitted to the intensive care unit (ICU) in two tertiary hospitals in Saudi Arabia were retrospectively assessed for zinc use from March 1, 2020 until March 31, 2021. After propensity score matching (1:1 ratio) based on the selected criteria, we assessed the association of zinc used as adjunctive therapy with the 30-day mortality. Secondary outcomes included the in-hospital mortality, ventilator free days, ICU length of stay (LOS), hospital LOS, and complication (s) during ICU stay.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 164 patients were included, 82 patients received zinc. Patients who received zinc sulfate as adjunctive therapy have a lower 30-day mortality (HR 0.52, CI 0.29, 0.92; <jats:italic>p</jats:italic> = 0.03). On the other hand, the in-hospital mortality was not statistically significant between the two groups (HR 0.64, CI 0.37–1.10; <jats:italic>p</jats:italic> = 0.11). Zinc sulfate use was associated with a lower odds of acute kidney injury development during ICU stay (OR 0.46 CI 0.19–1.06; <jats:italic>p</jats:italic> = 0.07); however, it did not reach statistical significance.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The use of zinc sulfate as an additional treatment in critically ill COVID-19 patients may improve survival. Furthermore, zinc supplementation may have a protective effect on the kidneys.</jats:p>\n </jats:sec>",
"alternative-id": [
"3785"
],
"article-number": "363",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2 June 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "6 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "18 October 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved in November 19th, 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Reference No: RC20/589/R). Participants' confidentiality was strictly observed throughout the study using the anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5547-2043",
"affiliation": [],
"authenticated-orcid": false,
"family": "Al Sulaiman",
"given": "Khalid",
"sequence": "first"
},
{
"affiliation": [],
"family": "Aljuhani",
"given": "Ohoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Shaya",
"given": "Abdulrahman I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kharbosh",
"given": "Abdullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kensara",
"given": "Raed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Guwairy",
"given": "Alhomaidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alharbi",
"given": "Aisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Algarni",
"given": "Rahmah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Harbi",
"given": "Shmeylan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vishwakarma",
"given": "Ramesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Korayem",
"given": "Ghazwa B.",
"sequence": "additional"
}
],
"container-title": "Critical Care",
"container-title-short": "Crit Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
18
]
],
"date-time": "2021-10-18T22:54:38Z",
"timestamp": 1634597678000
},
"deposited": {
"date-parts": [
[
2021,
10,
18
]
],
"date-time": "2021-10-18T23:11:24Z",
"timestamp": 1634598684000
},
"indexed": {
"date-parts": [
[
2024,
2,
19
]
],
"date-time": "2024-02-19T13:47:08Z",
"timestamp": 1708350428179
},
"is-referenced-by-count": 16,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
10,
18
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
18
]
],
"date-time": "2021-10-18T00:00:00Z",
"timestamp": 1634515200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
18
]
],
"date-time": "2021-10-18T00:00:00Z",
"timestamp": 1634515200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-021-03785-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13054-021-03785-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-021-03785-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
10,
18
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
18
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "The Lancet",
"key": "3785_CR1",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"author": "D Wang",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "JAMA",
"key": "3785_CR2",
"unstructured": "Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2141",
"author": "EA Coomes",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Rev Med Virol",
"key": "3785_CR3",
"unstructured": "Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1–9.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.02.037",
"author": "KA al Sulaiman",
"doi-asserted-by": "publisher",
"first-page": "180",
"journal-title": "Int J Infect Dis",
"key": "3785_CR4",
"unstructured": "al Sulaiman KA, Aljuhani O, Eljaaly K, Alharbi AA, al Shabasy AM, Alsaeedi AS, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–7.",
"volume": "105",
"year": "2021"
},
{
"author": "S Abd-Elsalam",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "3785_CR5",
"unstructured": "Abd-Elsalam S, Soliman S, Esmail ES, Khalaf M, Mostafa EF, Medhat MA, et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine? A randomized multicenter trial. Biol Trace Elem Res. 2020;25:1–5.",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "854",
"journal-title": "Intensive Care Med",
"key": "3785_CR6",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87. https://doi.org/10.1007/s00134-020-06022-5.",
"volume": "46",
"year": "2020"
},
{
"author": "W Alhazzani",
"first-page": "E219",
"journal-title": "Critical Care Med",
"key": "3785_CR7",
"unstructured": "Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Critical Care Med. 2019;2021:E219–34.",
"volume": "2021",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "The RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "3785_CR8",
"unstructured": "The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2020.6252",
"author": "S Gupta",
"doi-asserted-by": "publisher",
"first-page": "41",
"journal-title": "JAMA Intern Med",
"key": "3785_CR9",
"unstructured": "Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51.",
"volume": "181",
"year": "2021"
},
{
"author": "VC Veiga",
"first-page": "372",
"journal-title": "BMJ",
"key": "3785_CR10",
"unstructured": "Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2019;2021:372.",
"volume": "2021",
"year": "2019"
},
{
"author": "MM Aleissa",
"first-page": "e01814",
"journal-title": "Antimicrob Agents Chemother",
"key": "3785_CR11",
"unstructured": "Aleissa MM, Silverman EA, Paredes Acosta LM, Nutt CT, Richterman A, Marty FM. New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother. 2021;65:e01814.",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.maturitas.2020.08.003",
"author": "H Shakoor",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Maturitas",
"key": "3785_CR12",
"unstructured": "Shakoor H, Feehan J, al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9.",
"volume": "143",
"year": "2021"
},
{
"DOI": "10.1093/advances/nmz013",
"author": "SA Read",
"doi-asserted-by": "publisher",
"first-page": "696",
"journal-title": "Adv Nutr",
"key": "3785_CR13",
"unstructured": "Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1177/095632020701800302",
"author": "DL Barnard",
"doi-asserted-by": "publisher",
"first-page": "125",
"journal-title": "Antiviral Chem Chemother",
"key": "3785_CR14",
"unstructured": "Barnard DL, Wong M-H, Bailey K, Day CW, Sidwell RW, Hickok SS, et al. Effect of oral gavage treatment with ZnAL42 and other metallo-ion formulations on influenza A H5N1 and H1N1 virus infections in mice. Antiviral Chem Chemother. 2007;18:125–32.",
"volume": "18",
"year": "2007"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"author": "AJW te Velthuis",
"doi-asserted-by": "publisher",
"first-page": "e1001176",
"journal-title": "PLoS Pathog",
"key": "3785_CR15",
"unstructured": "te Velthuis AJW, van den Worml SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:e1001176.",
"volume": "6",
"year": "2010"
},
{
"DOI": "10.3892/ijmm.2020.4790",
"author": "A Pormohammad",
"doi-asserted-by": "publisher",
"first-page": "326",
"journal-title": "Int J Mol Med",
"key": "3785_CR16",
"unstructured": "Pormohammad A, Monych NK, Turner RJ, Pormohammad A. Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int J Mol Med. 2021;47:326–34.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.109848",
"author": "A Kumar",
"doi-asserted-by": "publisher",
"first-page": "109848",
"journal-title": "Med Hypotheses",
"key": "3785_CR17",
"unstructured": "Kumar A, Kubota Y, Chernov M, Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144:109848.",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106214",
"author": "R Derwand",
"doi-asserted-by": "publisher",
"first-page": "106214",
"journal-title": "Int J Antimicrob Agents",
"key": "3785_CR18",
"unstructured": "Derwand R, Scholz M, Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56:106214.",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1177/0148607108322402",
"author": "DK Heyland",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "J Parenter Enter Nutr",
"key": "3785_CR19",
"unstructured": "Heyland DK, Jones N, Cvijanovich NZ, Wong H. Review: zinc supplementation in critically ill patients: a key pharmaconutrient? J Parenter Enter Nutr. 2008;32:509–19.",
"volume": "32",
"year": "2008"
},
{
"key": "3785_CR20",
"unstructured": "MDcalc. MDcalc-Medical calculators, equations, scores, and guidelines. [Internet]. [cited 2021 May 28]. Available from: MDcalc.com"
},
{
"DOI": "10.1038/kisup.2011.32",
"doi-asserted-by": "crossref",
"key": "3785_CR21",
"unstructured": "Kidney Disease: Improving Global Outcomes (KDIGO). Section 2: AKI Definition. Kidney International Supplements. 2012;2:19–36."
},
{
"key": "3785_CR22",
"unstructured": "ICD - ICD-10-CM: International Classification of Diseases, Tenth Revision, Clinical Modification. 2021."
},
{
"DOI": "10.1101/2020.05.02.20080036",
"doi-asserted-by": "crossref",
"key": "3785_CR23",
"unstructured": "Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, Rahimian J. Hydroxychloroquine and azithromycin plus zinc vs. hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients. J Medical Microbiol. 2020;1228–34."
},
{
"DOI": "10.20452/pamw.16048",
"doi-asserted-by": "crossref",
"key": "3785_CR24",
"unstructured": "Szarpak L, Pruc M, Gasecka A, Jaguszewski MJ, Michalski T, Peacock FW, et al. Should we supplement zinc in COVID-19 patients? Evidence from meta-analysis. Polish Arch Intern Med. 2021."
},
{
"DOI": "10.1093/cid/ciaa449",
"author": "X Chen",
"doi-asserted-by": "publisher",
"first-page": "1937",
"journal-title": "Clin Infect Dis",
"key": "3785_CR25",
"unstructured": "Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020;71:1937–42.",
"volume": "71",
"year": "2020"
},
{
"author": "IA Al-Saleh",
"first-page": "37",
"journal-title": "J Environ Pathol Toxicol Oncol",
"key": "3785_CR26",
"unstructured": "Al-Saleh IA, Al-Jaloud A, Al-Doush I, El-Din G. The distribution of selenium levels in Saudi dairy farms: a preliminary report from Al-Kharj. J Environ Pathol Toxicol Oncol. 1999;18:37–46.",
"volume": "18",
"year": "1999"
},
{
"DOI": "10.1016/j.clnu.2021.04.042",
"author": "J Fromonot",
"doi-asserted-by": "publisher",
"journal-title": "Clin Nutr",
"key": "3785_CR27",
"unstructured": "Fromonot J, Gette M, Ben Lassoued A, Guéant JL, Guéant-Rodriguez RM, Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin Nutr. 2021. https://doi.org/10.1016/j.clnu.2021.04.042.",
"year": "2021"
},
{
"DOI": "10.1097/CCM.0000000000004917",
"author": "SAP Short",
"doi-asserted-by": "publisher",
"first-page": "e500",
"journal-title": "Crit Care Med",
"key": "3785_CR28",
"unstructured": "Short SAP, Gupta S, Brenner SK, Hayek SS, Srivastava A, Shaefi S, et al. D-dimer and Death in critically ill patients with coronavirus disease 2019. Crit Care Med. 2021;49:e500.",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-040580",
"author": "M Perera",
"doi-asserted-by": "publisher",
"first-page": "e040580",
"journal-title": "BMJ Open",
"key": "3785_CR29",
"unstructured": "Perera M, el Khoury J, Chinni V, Bolton D, Qu L, Johnson P, et al. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 2020;10:e040580.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1177/1753466620937175",
"author": "I Huang",
"doi-asserted-by": "publisher",
"first-page": "175346662093717",
"journal-title": "Ther Adv Respir Dis",
"key": "3785_CR30",
"unstructured": "Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. https://doi.org/10.1177/1753466620937175.",
"volume": "14",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://ccforum.biomedcentral.com/articles/10.1186/s13054-021-03785-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}
