Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study
et al., Critical Care, doi:10.1186/s13054-022-04158-y, Oct 2022
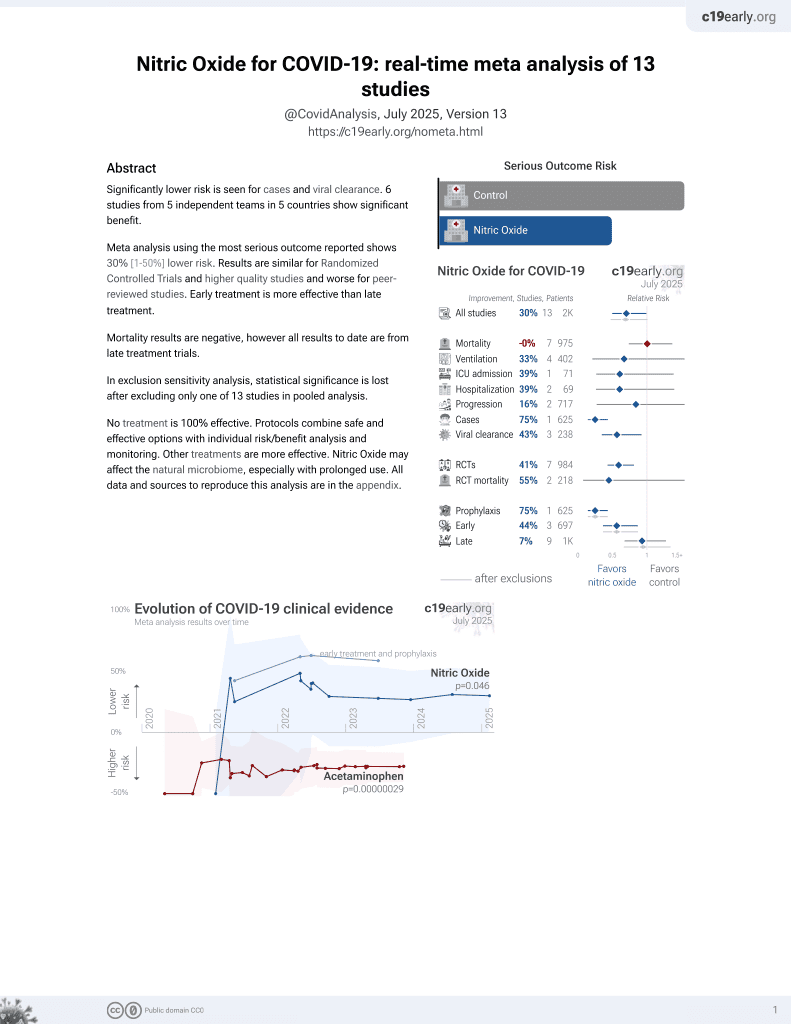
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
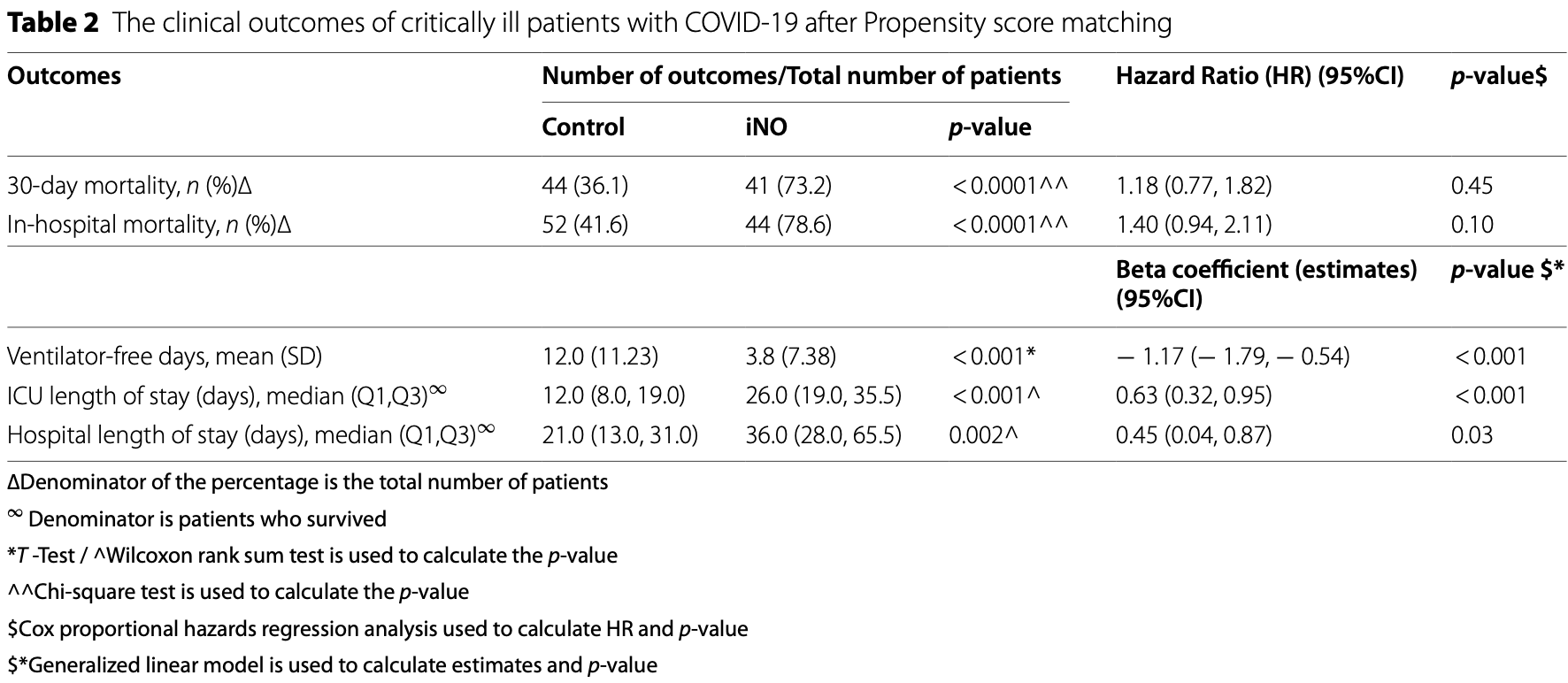
Retrospective 815 COVID-19 ICU patients in Saudi Arabia, showing significant improvement in oxygenation within 24 hours with inhaled nitric oxide (iNO). There was no significant difference in mortality, and ICU and hospitalization time was longer.
Data in this study is not clear - the reported mortality percentages are inconsistent with the stated PS-matched sample sizes. For example, the 30-day mortality of 41/70 in the treatment group is 58.6%, but the paper reports 73.2%.
Results are unreliable due to confounding by indication and immortal time bias. The study states that iNO was used "as rescue therapy in patients with refractory hypoxemia" and administration was a median of 6 days after admission (no IQR provided). A patient receiving iNO had failed to respond to a median of 6 days of standard ARDS management, and had persistent severe hypoxemia. This creates immortal time bias but also severe confounding by indication, for which the propensity score matching has only minimal effect. This is because the authors perform matching at baseline, but then compare treatment initiated based on the condition a median 6 days later. They are asking: among patients who looked similar at ICU admission, did those who eventually received rescue therapy with iNO have different outcomes? A better question would be: among patients who reached a specified point of refractory hypoxemia despite standard care, did iNO improve outcomes compared to continued standard care? To illustrate this, consider two patients that had baseline SOFA 5. One patient progresses to SOFA 9 and starts rescue therapy on day 6, while the other improves daily and is discharged at day 6. Comparing these patients is not informative for the efficacy of iNO.
Two important prognostic variables show statistically significant imbalance after PS matching: C-reactive protein and liver disease. The higher liver injury outcome in the iNO group (OR 3.32, p=0.009) may be confounded by baseline liver disease prevalence.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
This study is excluded in meta-analysis:
substantial unadjusted confounding by indication likely; inconsistent data.
|
risk of death, 40.0% higher, HR 1.40, p = 0.10, treatment 44 of 56 (78.6%), control 52 of 125 (41.6%), adjusted per study, in-hospital mortality, multivariable, Cox proportional hazards.
|
|
risk of death, 18.0% higher, HR 1.18, p = 0.45, treatment 41 of 56 (73.2%), control 44 of 122 (36.1%), adjusted per study, multivariable, Cox proportional hazards, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Al Sulaiman et al., 3 Oct 2022, retrospective, Saudi Arabia, peer-reviewed, mean age 62.5, 29 authors, study period 1 March, 2020 - 31 July, 2021.
Contact: alsulaimankh@hotmail.com (corresponding author).
Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study
Critical Care, doi:10.1186/s13054-022-04158-y
Background: Inhaled nitric oxide (iNO) is used as rescue therapy in patients with refractory hypoxemia due to severe COVID-19 acute respiratory distress syndrome (ARDS) despite the recommendation against the use of this treatment. To date, the effect of iNO on the clinical outcomes of critically ill COVID-19 patients with moderate-to-severe ARDS remains arguable. Therefore, this study aimed to evaluate the use of iNO in critically ill COVID-19 patients with moderate-to-severe ARDS. Methods: This multicenter, retrospective cohort study included critically ill adult patients with confirmed COVID-19 treated from March 01, 2020, until July 31, 2021. Eligible patients with moderate-to-severe ARDS were subsequently categorized into two groups based on inhaled nitric oxide (iNO) use throughout their ICU stay. The primary endpoint was the improvement in oxygenation parameters 24 h after iNO use. Other outcomes were considered secondary. Propensity score matching (1:2) was used based on the predefined criteria. Results: A total of 1598 patients were screened, and 815 were included based on the eligibility criteria. Among them, 210 patients were matched based on predefined criteria. Oxygenation parameters (PaO 2 , FiO 2 requirement, P/F ratio, oxygenation index) were significantly improved 24 h after iNO administration within a median of six days of ICU admission. However, the risk of 30-day and in-hospital mortality were found to be similar between the two groups (HR: 1.18; 95% CI: 0.77, 1.82; p = 0.45 and HR: 1.40; 95% CI: 0.94, 2.11; p= 0.10, respectively). On the other hand, ventilator-free days (VFDs) were significantly fewer, and ICU and hospital LOS were significantly longer in the iNO group.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13054-022-04158-y.
Additional file 1. Outcomes definition(s).
Author contributions All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate The study was approved in December 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Ref.# RC20.638.R). Participants' confidentiality was strictly observed throughout the study by using anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center.
Consent for publication Not applicable.
Competing interests No author has a conflict of interest in this study. • fast, convenient online submission • thorough peer review by experienced researchers in your field
• rapid publication on acceptance • support for..
References
Abdullah, None
Akaike, Maeda, Nitric oxide and virus infection, Immunology
Aleidan, Alkhelaifi, Alsenaid, Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: a matched case-control study, Expert Rev Anti Infect Ther
Alhazzani, Møller, Arabi, Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19), Intensive Care Med
Aslan, Aslan, Zolbanin, Acute respiratory distress syndrome in COVID-19: possible mechanisms and therapeutic management, Pneumonia
Bateman, Sharpe, Jagger, 36th International Symposium on Intensive Care and Emergency Medicine, Crit Care
Beran, Mhanna, Srour, Inhaled pulmonary vasodilator treatment for COVID-19: a systematic review and meta-analysis, Chest
Bhat, Neuman, Tantary, Inhaled nitric oxide in acute pulmonary embolism: a systematic review, Rev Cardiovasc Med
Dellinger, Zimmerman, Taylor, Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group, Crit Care Med
Diblasi, Myers, Hess, Evidence-based clinical practice guideline: inhaled nitric oxide for neonates with acute hypoxic respiratory failure, Respir Care
Fakhr, Fenza, Gianni, Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia, Nitric oxide Biol Chem
Fan, Brodie, Slutsky, Acute respiratory distress syndrome: advances in diagnosis and treatment, JAMA
Ferrari, Santini, Protti, Inhaled nitric oxide in mechanically ventilated patients with COVID-19, J Crit Care
Garfield, Mcfadyen, Briar, Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia, Br J Anaesth
Gebistorf, Karam, Wetterslev, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults, Cochrane Database Syst Rev
Gentile, Inhaled medical gases: more to breathe than oxygen, Respir Care
Grasselli, Cattaneo, Florio, Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review, Crit Care
Grasselli, Zangrillo, Zanella, Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy JAMA
Hirsch, Ng, Ross, Acute kidney injury in patients hospitalized with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.05.006
Hoste, Bagshaw, Bellomo, Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study, Intensive Care Med
Jain, Dalnogare, Pharmacological therapy for acute respiratory distress syndrome, Mayo Clin Proc
Karam, Gebistorf, Wetterslev, The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a cochrane systematic review with trial sequential analysis, Anaesthesia
Koyfman, Simchon, Koyfman, Clinical outcomes of critically ill patients using inhaled nitric oxide (iNO) during intrahospital transport, Crit Care Res Pract
Kumar, Arora, Sharma, Is diabetes mellitus associated with mortality and severity of COVID-19? Meta Anal Diabetes, Metab Syndr
Lim, Subramaniam, Reddy, Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis, Am J Respir Crit Care Med
Lin, Chen, Acute kidney injury classification: AKIN and RIFLE criteria in critical patients, World J Crit care Med
Longobardo, Montanari, Shulman, Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome, Br J Anaesth
Lotz, Muellenbach, Meybohm, Effects of inhaled nitric oxide in COVID-19-induced ARDS -Is it worthwhile?, Acta Anaesthesiol Scand
Norderfeldt, Liliequist, Frostell, Acute pulmonary hypertension and short-term outcomes in severe Covid-19 patients needing intensive care, Acta Anaesthesiol Scand, doi:10.1111/aas.13819
Oliveira, Parikh, Lopez-Ruiz, ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida, PLoS ONE
Page, Ariëns, Mechanisms of thrombosis and cardiovascular complications in COVID-19, Thromb Res
Parikh, Wilson, Weinberg, Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients, Ther Adv Respir Dis
Prakash, Kaur, Kaur, Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review, Indian J Pharmacol
Rodriguez-Roisin, Pulmonary gas exchange in acute respiratory failure, Eur J Anaesthesiol
Sulaiman, Aljuhani, Eljaaly, Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study, Int J Infect Dis, doi:10.1016/j.ijid.2021.02.037
Swenson, Swenson, Pathophysiology of acute Respiratory distress syndrome and COVID-19 lung injury, Crit Care Clin
Tavazzi, Pozzi, Mongodi, Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia, Crit Care
Taylor, Zimmerman, Dellinger, Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial, JAMA
Wang, Cong, Miao, Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials, Ren Fail
Wang, Lu, Li, Clinical course and outcomes of 344 intensive care patients with COVID-19, Am J Respir Crit Care Med
Weinberger, Laskin, Heck, Laskin, The toxicology of inhaled nitric oxide, Toxicol Sci, doi:10.1093/toxsci/59.1.5
Welker, Huang, Gil, acute respiratory distress syndrome update, with coronavirus disease 2019 focus, J Cardiothorac Vasc Anesth
Wilcox, Management of respiratory failure due to covid-19, BMJ
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study, Lancet Respir Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
DOI record:
{
"DOI": "10.1186/s13054-022-04158-y",
"ISSN": [
"1364-8535"
],
"URL": "http://dx.doi.org/10.1186/s13054-022-04158-y",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Inhaled nitric oxide (iNO) is used as rescue therapy in patients with refractory hypoxemia due to severe COVID-19 acute respiratory distress syndrome (ARDS) despite the recommendation against the use of this treatment. To date, the effect of iNO on the clinical outcomes of critically ill COVID-19 patients with moderate-to-severe ARDS remains arguable. Therefore, this study aimed to evaluate the use of iNO in critically ill COVID-19 patients with moderate-to-severe ARDS.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This multicenter, retrospective cohort study included critically ill adult patients with confirmed COVID-19 treated from March 01, 2020, until July 31, 2021. Eligible patients with moderate-to-severe ARDS were subsequently categorized into two groups based on inhaled nitric oxide (iNO) use throughout their ICU stay. The primary endpoint was the improvement in oxygenation parameters 24 h after iNO use. Other outcomes were considered secondary. Propensity score matching (1:2) was used based on the predefined criteria.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 1598 patients were screened, and 815 were included based on the eligibility criteria. Among them, 210 patients were matched based on predefined criteria. Oxygenation parameters (PaO<jats:sub>2</jats:sub>, FiO<jats:sub>2</jats:sub> requirement, P/F ratio, oxygenation index) were significantly improved 24 h after iNO administration within a median of six days of ICU admission. However, the risk of 30-day and in-hospital mortality were found to be similar between the two groups (HR: 1.18; 95% CI: 0.77, 1.82; <jats:italic>p</jats:italic> = 0.45 and HR: 1.40; 95% CI: 0.94, 2.11; <jats:italic>p</jats:italic>= 0.10, respectively). On the other hand, ventilator-free days (VFDs) were significantly fewer, and ICU and hospital LOS were significantly longer in the iNO group. In addition, patients who received iNO had higher odds of acute kidney injury (AKI) (OR (95% CI): 2.35 (1.30, 4.26), <jats:italic>p</jats:italic> value = 0.005) and hospital/ventilator-acquired pneumonia (OR (95% CI): 3.2 (1.76, 5.83), <jats:italic>p</jats:italic> value = 0.001).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>In critically ill COVID-19 patients with moderate-to-severe ARDS, iNO rescue therapy is associated with improved oxygenation parameters but no mortality benefits. Moreover, iNO use is associated with higher odds of AKI, pneumonia, longer LOS, and fewer VFDs.</jats:p>\n </jats:sec>",
"alternative-id": [
"4158"
],
"article-number": "304",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "4 September 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "3 October 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was approved in December 2020 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Ref.# RC20.638.R). Participants’ confidentiality was strictly observed throughout the study by using anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "No author has a conflict of interest in this study."
}
],
"author": [
{
"affiliation": [],
"family": "Al Sulaiman",
"given": "Khalid",
"sequence": "first"
},
{
"affiliation": [],
"family": "Korayem",
"given": "Ghazwa B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Altebainawi",
"given": "Ali F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Harbi",
"given": "Shmeylan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alissa",
"given": "Abdulrahman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alharthi",
"given": "Abdullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kensara",
"given": "Raed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alfahed",
"given": "Amjaad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vishwakarma",
"given": "Ramesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Haji",
"given": "Hussain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almohaimid",
"given": "Naif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Zumai",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alrubayan",
"given": "Fahad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asiri",
"given": "Abdulmajid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkahtani",
"given": "Nasser",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alolayan",
"given": "Abdulaziz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsohimi",
"given": "Samiah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Melibari",
"given": "Nawal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almagthali",
"given": "Alaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aljahdali",
"given": "Seba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alenazi",
"given": "Abeer A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsaeedi",
"given": "Alawi S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Ghamdi",
"given": "Ghassan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Faris",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alqahtani",
"given": "Joud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al Qahtani",
"given": "Jalal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshammari",
"given": "Khalid A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshammari",
"given": "Khalil I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aljuhani",
"given": "Ohoud",
"sequence": "additional"
}
],
"container-title": "Critical Care",
"container-title-short": "Crit Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T11:02:41Z",
"timestamp": 1664794961000
},
"deposited": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T11:09:19Z",
"timestamp": 1664795359000
},
"indexed": {
"date-parts": [
[
2022,
10,
4
]
],
"date-time": "2022-10-04T05:05:01Z",
"timestamp": 1664859901583
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
10,
3
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T00:00:00Z",
"timestamp": 1664755200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
3
]
],
"date-time": "2022-10-03T00:00:00Z",
"timestamp": 1664755200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-022-04158-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13054-022-04158-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-022-04158-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
10,
3
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
3
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "4158_CR1",
"unstructured": "Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [Internet]. Lancet. 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1786",
"author": "SR Wilcox",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "4158_CR2",
"unstructured": "Wilcox SR. Management of respiratory failure due to covid-19. BMJ. 2020;369: m1786.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202006-2405OC",
"author": "ZJ Lim",
"doi-asserted-by": "publisher",
"first-page": "54",
"journal-title": "Am J Respir Crit Care Med",
"key": "4158_CR3",
"unstructured": "Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis. Am J Respir Crit Care Med. 2021;203:54–66.",
"volume": "203",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0249038",
"author": "E Oliveira",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "4158_CR4",
"unstructured": "Oliveira E, Parikh A, Lopez-Ruiz A, et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS ONE. 2021;16: e0249038.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1186/s13054-021-03536-2",
"author": "G Grasselli",
"doi-asserted-by": "publisher",
"first-page": "115",
"journal-title": "Crit Care",
"key": "4158_CR5",
"unstructured": "Grasselli G, Cattaneo E, Florio G, et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care. 2021;25:115.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.5394",
"author": "G Grasselli",
"doi-asserted-by": "publisher",
"first-page": "1574",
"journal-title": "Italy JAMA",
"key": "4158_CR6",
"unstructured": "Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy JAMA. 2020;323:1574–81.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0736LE",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1430",
"journal-title": "Am J Respir Crit Care Med",
"key": "4158_CR7",
"unstructured": "Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201:1430–4.",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"author": "X Yang",
"doi-asserted-by": "publisher",
"first-page": "475",
"journal-title": "Lancet Respir Med",
"key": "4158_CR8",
"unstructured": "Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1186/s41479-021-00092-9",
"author": "A Aslan",
"doi-asserted-by": "publisher",
"first-page": "14",
"journal-title": "Pneumonia (Nathan Qld)",
"key": "4158_CR9",
"unstructured": "Aslan A, Aslan C, Zolbanin NM, et al. Acute respiratory distress syndrome in COVID-19: possible mechanisms and therapeutic management. Pneumonia (Nathan Qld). 2021;13:14.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.4065/81.2.205",
"author": "R Jain",
"doi-asserted-by": "publisher",
"first-page": "205",
"journal-title": "Mayo Clin Proc",
"key": "4158_CR10",
"unstructured": "Jain R, DalNogare A. Pharmacological therapy for acute respiratory distress syndrome. Mayo Clin Proc. 2006;81:205–12.",
"volume": "81",
"year": "2006"
},
{
"DOI": "10.1001/jama.2017.21907",
"author": "E Fan",
"doi-asserted-by": "publisher",
"first-page": "698",
"journal-title": "JAMA",
"key": "4158_CR11",
"unstructured": "Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710.",
"volume": "319",
"year": "2018"
},
{
"DOI": "10.1016/j.ccc.2021.05.003",
"author": "KE Swenson",
"doi-asserted-by": "publisher",
"first-page": "749",
"journal-title": "Crit Care Clin",
"key": "4158_CR12",
"unstructured": "Swenson KE, Swenson ER. Pathophysiology of acute Respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37:749–76.",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1053/j.jvca.2021.02.053",
"author": "C Welker",
"doi-asserted-by": "publisher",
"first-page": "1188",
"journal-title": "J Cardiothorac Vasc Anesth",
"key": "4158_CR13",
"unstructured": "Welker C, Huang J, Gil IJN, et al. 2021 acute respiratory distress syndrome update, with coronavirus disease 2019 focus. J Cardiothorac Vasc Anesth. 2021;36:1188.",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1097/00003246-199801000-00011",
"author": "RP Dellinger",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "Crit Care Med",
"key": "4158_CR14",
"unstructured": "Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group. Crit Care Med. 1998;26:15–23.",
"volume": "26",
"year": "1998"
},
{
"author": "F Gebistorf",
"first-page": "CD002787",
"journal-title": "Cochrane Database Syst Rev",
"key": "4158_CR15",
"unstructured": "Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016;2016:CD002787.",
"volume": "2016",
"year": "2016"
},
{
"DOI": "10.1016/j.niox.2021.08.003",
"author": "B Safaee Fakhr",
"doi-asserted-by": "publisher",
"first-page": "7",
"journal-title": "Nitric oxide Biol Chem",
"key": "4158_CR16",
"unstructured": "Safaee Fakhr B, Di Fenza R, Gianni S, et al. Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia. Nitric oxide Biol Chem. 2021;116:7–13.",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1111/aas.13757",
"author": "C Lotz",
"doi-asserted-by": "publisher",
"first-page": "629",
"journal-title": "Acta Anaesthesiol Scand",
"key": "4158_CR17",
"unstructured": "Lotz C, Muellenbach RM, Meybohm P, et al. Effects of inhaled nitric oxide in COVID-19-induced ARDS - Is it worthwhile? Acta Anaesthesiol Scand. 2021;65:629–32.",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.bja.2020.11.006",
"author": "B Garfield",
"doi-asserted-by": "publisher",
"first-page": "e72",
"journal-title": "Br J Anaesth",
"key": "4158_CR18",
"unstructured": "Garfield B, McFadyen C, Briar C, et al. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br J Anaesth. 2021;126:e72–5.",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03222-9",
"author": "G Tavazzi",
"doi-asserted-by": "publisher",
"first-page": "508",
"journal-title": "Crit Care",
"key": "4158_CR19",
"unstructured": "Tavazzi G, Pozzi M, Mongodi S, et al. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24:508.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2021.07.539",
"author": "A Beran",
"doi-asserted-by": "publisher",
"first-page": "a558",
"journal-title": "Chest",
"key": "4158_CR20",
"unstructured": "Beran A, Mhanna M, Srour O, et al. Inhaled pulmonary vasodilator treatment for COVID-19: a systematic review and meta-analysis. Chest. 2021;160:a558.",
"volume": "160",
"year": "2021"
},
{
"key": "4158_CR21",
"unstructured": "COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. [Internet]. [cited 2022 Jan 21] Available from: https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "854",
"journal-title": "Intensive Care Med",
"key": "4158_CR22",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46:854–87.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.4187/respcare.01442",
"author": "MA Gentile",
"doi-asserted-by": "publisher",
"first-page": "1341",
"journal-title": "Respir Care",
"key": "4158_CR23",
"unstructured": "Gentile MA. Inhaled medical gases: more to breathe than oxygen. Respir Care. 2011;56:1341–9.",
"volume": "56",
"year": "2011"
},
{
"author": "RM DiBlasi",
"first-page": "1717",
"journal-title": "Respir Care",
"key": "4158_CR24",
"unstructured": "DiBlasi RM, Myers TR, Hess DR. Evidence-based clinical practice guideline: inhaled nitric oxide for neonates with acute hypoxic respiratory failure. Respir Care. 2010;55:1717–45.",
"volume": "55",
"year": "2010"
},
{
"DOI": "10.1093/toxsci/59.1.5",
"author": "B Weinberger",
"doi-asserted-by": "publisher",
"first-page": "5",
"issue": "1",
"journal-title": "Toxicol Sci",
"key": "4158_CR25",
"unstructured": "Weinberger B, Laskin DL, Heck DE, Laskin JD. The toxicology of inhaled nitric oxide. Toxicol Sci. 2001;59(1):5–16. https://doi.org/10.1093/toxsci/59.1.5.",
"volume": "59",
"year": "2001"
},
{
"DOI": "10.5492/wjccm.v1.i2.40",
"author": "C-Y Lin",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "World J Crit care Med",
"key": "4158_CR26",
"unstructured": "Lin C-Y, Chen Y-C. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J Crit care Med. 2012;1:40–5.",
"volume": "1",
"year": "2012"
},
{
"DOI": "10.1080/14787210.2020.1822736",
"author": "FAS Aleidan",
"doi-asserted-by": "publisher",
"first-page": "393",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "4158_CR27",
"unstructured": "Aleidan FAS, Alkhelaifi H, Alsenaid A, et al. Incidence and risk factors of carbapenem-resistant Enterobacteriaceae infection in intensive care units: a matched case-control study. Expert Rev Anti Infect Ther. 2021;19:393–8.",
"volume": "19",
"year": "2021"
},
{
"author": "R Rodriguez-Roisin",
"first-page": "5",
"journal-title": "Eur J Anaesthesiol",
"key": "4158_CR28",
"unstructured": "Rodriguez-Roisin R. Pulmonary gas exchange in acute respiratory failure. Eur J Anaesthesiol. 1994;11:5–13.",
"volume": "11",
"year": "1994"
},
{
"DOI": "10.1016/j.kint.2020.05.006",
"author": "JS Hirsch",
"doi-asserted-by": "publisher",
"first-page": "209",
"issue": "1",
"journal-title": "Kidney Int",
"key": "4158_CR29",
"unstructured": "Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18. https://doi.org/10.1016/j.kint.2020.05.006.",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.02.037",
"author": "KA Al Sulaiman",
"doi-asserted-by": "publisher",
"first-page": "180",
"journal-title": "Int J Infect Dis",
"key": "4158_CR30",
"unstructured": "Al Sulaiman KA, Aljuhani O, Eljaaly K, et al. Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study. Int J Infect Dis. 2021;105:180–7. https://doi.org/10.1016/j.ijid.2021.02.037.",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1111/aas.13819",
"author": "J Norderfeldt",
"doi-asserted-by": "publisher",
"first-page": "761",
"issue": "6",
"journal-title": "Acta Anaesthesiol Scand",
"key": "4158_CR31",
"unstructured": "Norderfeldt J, Liliequist A, Frostell C, et al. Acute pulmonary hypertension and short-term outcomes in severe Covid-19 patients needing intensive care. Acta Anaesthesiol Scand. 2021;65(6):761–9. https://doi.org/10.1111/aas.13819.",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.jcrc.2020.08.007",
"author": "M Ferrari",
"doi-asserted-by": "publisher",
"first-page": "159",
"journal-title": "J Crit Care",
"key": "4158_CR32",
"unstructured": "Ferrari M, Santini A, Protti A, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–60.",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1177/1753466620933510",
"author": "R Parikh",
"doi-asserted-by": "publisher",
"first-page": "175346662093351",
"journal-title": "Ther Adv Respir Dis",
"key": "4158_CR33",
"unstructured": "Parikh R, Wilson C, Weinberg J, et al. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther Adv Respir Dis. 2020;14:1753466620933510.",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.4103/ijp.ijp_901_20",
"author": "A Prakash",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "Indian J Pharmacol",
"key": "4158_CR34",
"unstructured": "Prakash A, Kaur S, Kaur C, et al. Efficacy and safety of inhaled nitric oxide in the treatment of severe/critical COVID-19 patients: a systematic review. Indian J Pharmacol. 2021;53:236–43.",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1111/anae.13628",
"author": "O Karam",
"doi-asserted-by": "publisher",
"first-page": "106",
"journal-title": "Anaesthesia",
"key": "4158_CR35",
"unstructured": "Karam O, Gebistorf F, Wetterslev J, et al. The effect of inhaled nitric oxide in acute respiratory distress syndrome in children and adults: a cochrane systematic review with trial sequential analysis. Anaesthesia. 2017;72:106–17.",
"volume": "72",
"year": "2017"
},
{
"DOI": "10.1016/j.bja.2020.10.011",
"author": "A Longobardo",
"doi-asserted-by": "publisher",
"first-page": "e44",
"journal-title": "Br J Anaesth",
"key": "4158_CR36",
"unstructured": "Longobardo A, Montanari C, Shulman R, et al. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021;126:e44–6.",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1007/s00134-015-3934-7",
"author": "EAJ Hoste",
"doi-asserted-by": "publisher",
"first-page": "1411",
"journal-title": "Intensive Care Med",
"key": "4158_CR37",
"unstructured": "Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23.",
"volume": "41",
"year": "2015"
},
{
"DOI": "10.1016/j.dsx.2020.04.044",
"author": "A Kumar",
"doi-asserted-by": "publisher",
"first-page": "535",
"journal-title": "Meta Anal Diabetes Metab Syndr",
"key": "4158_CR38",
"unstructured": "Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? Meta Anal Diabetes Metab Syndr. 2020;14:535–45.",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1001/jama.291.13.1603",
"author": "RW Taylor",
"doi-asserted-by": "publisher",
"first-page": "1603",
"journal-title": "JAMA",
"key": "4158_CR39",
"unstructured": "Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004;291:1603–9.",
"volume": "291",
"year": "2004"
},
{
"DOI": "10.1080/0886022X.2021.1873805",
"author": "J Wang",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Ren Fail",
"key": "4158_CR40",
"unstructured": "Wang J, Cong X, Miao M, et al. Inhaled nitric oxide and acute kidney injury risk: a meta-analysis of randomized controlled trials. Ren Fail. 2021;43:281–90.",
"volume": "43",
"year": "2021"
},
{
"author": "L Koyfman",
"first-page": "6633210",
"journal-title": "Crit Care Res Pract",
"key": "4158_CR41",
"unstructured": "Koyfman L, Simchon O, Koyfman A, et al. Clinical outcomes of critically ill patients using inhaled nitric oxide (iNO) during intrahospital transport. Crit Care Res Pract. 2021;2021:6633210.",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1016/j.thromres.2021.01.005",
"author": "EM Page",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Thromb Res",
"key": "4158_CR42",
"unstructured": "Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. 2021;200:1–8.",
"volume": "200",
"year": "2021"
},
{
"DOI": "10.3909/ricm0718",
"author": "T Bhat",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Rev Cardiovasc Med",
"key": "4158_CR43",
"unstructured": "Bhat T, Neuman A, Tantary M, et al. Inhaled nitric oxide in acute pulmonary embolism: a systematic review. Rev Cardiovasc Med. 2015;16:1–8.",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1046/j.1365-2567.2000.00142.x",
"author": "T Akaike",
"doi-asserted-by": "publisher",
"first-page": "300",
"journal-title": "Immunology",
"key": "4158_CR44",
"unstructured": "Akaike T, Maeda H. Nitric oxide and virus infection. Immunology. 2000;101:300–8.",
"volume": "101",
"year": "2000"
},
{
"DOI": "10.1186/s13054-016-1208-6",
"doi-asserted-by": "crossref",
"key": "4158_CR45",
"unstructured": "Bateman RM, Sharpe MD, Jagger JE, et al. 36th International Symposium on Intensive Care and Emergency Medicine : Brussels, Belgium. 15–18 March 2016. Crit Care 2016; 20:94"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://ccforum.biomedcentral.com/articles/10.1186/s13054-022-04158-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "26"
}