The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkae045, PROSPERO CRD42023413208, Feb 2024
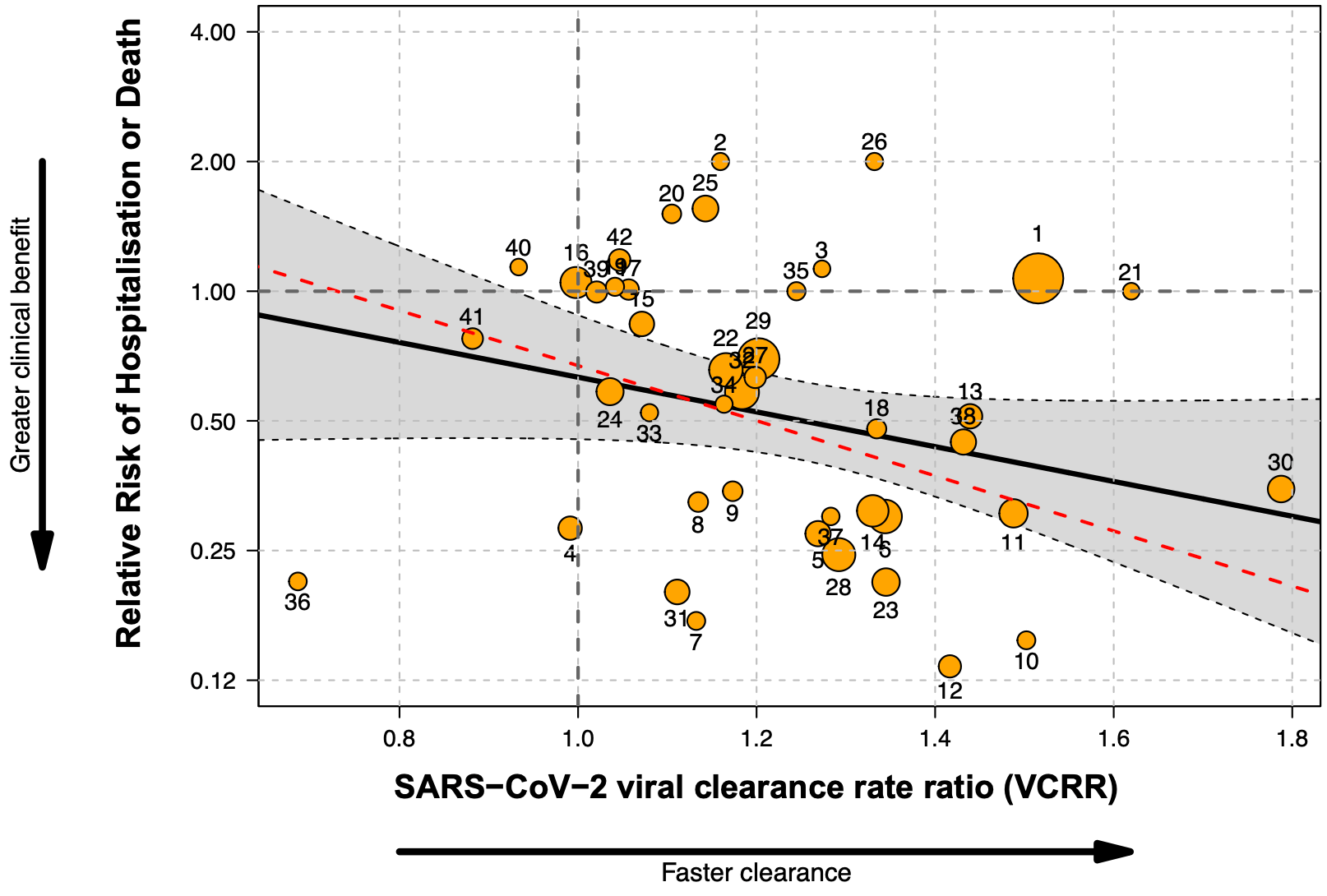
Systematic review and meta-analysis of 44 RCTs of antiviral treatments for early symptomatic COVID-19, including 52,384 participants, assessing the relationship between the SARS-CoV-2 viral clearance rate and the risk of hospitalization or death. After excluding one outlier trial, half of the variation (R2=50.4%) in clinical benefit was explained by differences in the viral clearance rate. Higher viral clearance rate was significantly associated with greater clinical benefit (lower risk of hospitalization/death).
Singh et al., 22 Feb 2024, Randomized Controlled Trial, placebo-controlled, Thailand, peer-reviewed, 6 authors, study period March 2020 - October 2022, trial PROSPERO CRD42023413208.
Contact: dr.simonboyd@gmail.com.
The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis
doi:10.1093/jac/dkae045/7612573
Background: Effective antiviral drugs accelerate viral clearance in acute COVID-19 infections; the relationship between accelerating viral clearance and reducing severe clinical outcomes is unclear. Methods: A systematic review was conducted of randomized controlled trials (RCTs) of antiviral therapies in early symptomatic COVID-19, where viral clearance data were available. Treatment benefit was defined clinically as the relative risk of hospitalization/death during follow-up (≥14 days), and virologically as the SARS-CoV-2 viral clearance rate ratio (VCRR). The VCRR is the ratio of viral clearance rates between the intervention and control arms. The relationship between the clinical and virological treatment effects was assessed by mixed-effects meta-regression.
Results: From 57 potentially eligible RCTs, VCRRs were derived for 44 (52 384 participants); 32 had ≥1 clinical endpoint in each arm. Overall, 9.7% (R 2 ) of the variation in clinical benefit was explained by variation in VCRRs with an estimated linear coefficient of -0.92 (95% CI: -1.99 to 0.13; P = 0.08). However, this estimate was highly sensitive to the inclusion of the recent very large PANORAMIC trial. Omitting this outlier, half the variation in clinical benefit (R 2 = 50.4%) was explained by variation in VCRRs [slope -1.47 (95% CI -2.43 to -0.51); P = 0.003], i.e. higher VCRRs were associated with an increased clinical benefit.
Conclusion: Methods of determining viral clearance in COVID-19 studies and the relationship to clinical outcomes vary greatly. As prohibitively large sample sizes are now required to show clinical treatment benefit in antiviral therapeutic assessments, viral clearance is a reasonable surrogate endpoint.
Transparency declarations The authors have none to report.
Supplementary data Supplementary methods and Tables S1 to S8 are available as Supplementary data at JAC Online.
References
Alemany, Millat-Martinez, Corbacho-Monné, High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00545-2
Batool, Vuthaluru, Hassan, Efficacy and safety of favipiravir in treating COVID-19 patients: a meta-analysis of randomized control trials, Cureus, doi:10.7759/cureus.33676
Bender, IM Tix-/Cil (57) 28, EPIC HR Nirmatrelvir/RTV
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Biber, Harmelin, The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19-a double-blind, randomized placebo-controlled trial, Int J Infect Dis, doi:10.1016/j.ijid.2022.07.003
Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet, doi:10.1016/S0140-6736(22)02597-1
Buyse, Molenberghs, Burzykowski, The validation of surrogate endpoints in meta-analyses of randomized experiments, Biostatistics, doi:10.1093/biostatistics/1.1.49
Chandiwana, Kruger, Johnstone, Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: a randomised, open-label, multi-arm, phase 2 clinical trial, eBioMedicine, doi:10.1016/j.ebiom.2022.104322
Charre, Levrero, Zoulim, Non-invasive biomarkers for chronic hepatitis B virus infection management, Antivir Res, doi:10.1016/j.antiviral.2019.104553
Cheema, Jafar, Elrashedy, Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients: a systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2022.10.012
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Chew, BLAZE-4 Bebtelovimab (53) 20
Chew, Moser, Daar, Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19, Nat Commun, doi:10.1038/s41467-022-32551-2
Cook, Detection of influential observation in linear regression, Technometrics, doi:10.2307/1268249
Dobler, Morgan, Falck-Ytter, Assessing the validity of surrogate endpoints in the context of a controversy about the measurement of effectiveness of hepatitis C virus treatment, BMJ Evid Based Med, doi:10.1136/bmjebm-2017-110852
Dougan, Azizad, Chen, Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19, medRxiv, doi:10.1101/2022.03.10.22272100
Dougan, Azizad, Mocherla, A randomized, placebocontrolled clinical trial of bamlanivimab and etesevimab together in highrisk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load, Clin Infect Dis, doi:10.1093/cid/ciab912
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Elias, Khan, Stadler, Viral clearance as a surrogate of clinical efficacy for COVID-19 therapies in outpatients: a systematic review and meta-analysis, medRxiv, doi:10.1101/2023.06.18.23291566
Erickson, Fenno, Barzilai, Metformin for treatment of acute COVID-19: systematic review of clinical trial data against SARS-CoV-2, Diabetes Care, doi:10.2337/dc22-2539
Evering, Amu/rom (31) 24, CoV-EARLY Conv.plasma
Evering, Chew, Giganti, Safety and efficacy of combination SARS-CoV-2 neutralizing monoclonal antibodies amubarvimab plus romlusevimab in nonhospitalized patients with COVID-19, Ann Intern Med, doi:10.7326/M22-3428
Feld, Kandel, Biondi, Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial, Lancet Respir Med, doi:10.1016/S2213-2600(20)30566-X
Feld, None, TOGETHER IFN
Fischer, Eron, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med, doi:10.1126/scitranslmed.abl7430
Fischer, None, Molnupiravir
Fischer, None, PINETREE Remdesivir
Ganesh, Pawlowski, Horo, Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19, J Clin Investig, doi:10.1172/JCI151697
Gharbharan, Jordans, Zwaginga, Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial, Clin Infect Dis, doi:10.1016/j.cmi.2022.08.005
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Grooth, Geenen, Girbes, SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis, Crit Care, doi:10.1186/s13054-017-1609-1
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19, JAMA, doi:10.1001/jama.2022.2832
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for highrisk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Ignacio, Chew, Moser, Safety and efficacy of combined tixagevimab and cilgavimab administered intramuscularly or intravenously in nonhospitalized patients with COVID-19: 2 randomized clinical trials, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.10039
Jadad, Moore, Carroll, Assessing the quality of reports of randomized clinical trials: is blinding necessary?, Control Clin Trials, doi:10.1016/0197-2456(95)00134-4
Jagannathan, Andrews, Bonilla, Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial, Nat Commun, doi:10.1038/s41467-021-22177-1
Jagannathan, None, COVID-OUT Metformin
Jittamala, Schilling, Watson, Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV), J Infect Dis, doi:10.1093/infdis/jiad275
Kaizer, Shapiro, Wild, Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2022.12.028
Kim, Sandulescu, Preotescu, A randomized clinical trial of regdanvimab in high-risk patients with mild-to-moderate coronavirus disease 2019, Open Forum Infect Dis, doi:10.1093/ofid/ofac406
Levine, Fukuta, Huaman, Coronavirus disease 2019 convalescent plasma outpatient therapy to prevent outpatient hospitalization: a meta-analysis of individual participant data from 5 randomized trials, Clin Infect Dis, doi:10.1093/cid/ciad088
Luvira, Schilling, Jittamala, Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial, BMC Infect Dis, doi:10.1186/s12879-023-08835-3
Martins-Filho, Do Nascimento-Junior, Barreto-Alves, Efficacy and safety of nitazoxanide in treating SARS-CoV-2 infection: a systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials, Eur J Clin Pharmacol, doi:10.1007/s00228-022-03380-5
Mcmahon, AR0-CORONA TDF, Favipiravir
Mcmahon, Lau, Coldham, Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101703
Mitja, None, CONV-ERT Conv. Plasma
Mitja, Reis, Boulware, Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: a meta-analysis of individual participant data of randomized trials, Clin Transl Sci, doi:10.1111/cts.13468
Mitjà, Corbacho-Monné, Ubals, Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial, Clin Infect Dis, doi:10.1093/cid/ciaa1009
Moher, Shamseer, Clarke, Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement, Syst Rev, doi:10.1186/2046-4053-4-1
Molina, Kennerley, Beaty, Real-world evaluation of bebtelovimab effectiveness during the period of COVID-19 omicron variants, including BA.4/BA.5, Int J Infect Dis, doi:10.1016/j.ijid.2023.04.396
Montgomery, Hobbs, Padilla, Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00180-1
Natori, Alghamdi, Tazari, Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis, Clin Infect Dis, doi:10.1093/cid/cix793
Parienti, De Grooth, Clinical relevance of nasopharyngeal SARS-CoV-2 viral load reduction in outpatients with COVID-19, J Antimicrob Chemother, doi:10.1093/jac/dkac104
Parienti, Prazuck, Paul, Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100993
Paules, Fauci, COVID-19: the therapeutic landscape, Med, doi:10.1016/j.medj.2021.04.015
Popp, Reis, Schiesser, Ivermectin for preventing and treating COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015017.pub3
Pujadas, Chaudhry, Mcbride, SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir Med, doi:10.1016/S2213-2600(20)30354-4
Reis, Silva, Silva, Early treatment with pegylated interferon lambda for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2209760
Rocco, Silva, Cruz, Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, Eur Respir J, doi:10.1183/13993003.03725-2020
Roozbeh, Saeedi, Alizadeh-Navaei, Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial, J Antimicrob Chemother, doi:10.1093/jac/dkaa501
Rossignol, Bardin, Fulgencio, A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101310
Schilling, Jittamala, Watson, Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00493-0
Schneider, Ayres, Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases, Nat Rev Immunol, doi:10.1038/nri2432
Shionogi, Shionogi presents pivotal ensitrelvir fumaric acid phase 3 data and exploratory long COVID data at CROI
Sinha, Kumarasamy, Suram, Efficacy and safety of molnupiravir in mild COVID-19 patients in India, Cureus, doi:10.7759/cureus.31508
Smith, Stein, Viral load as a surrogate end point in HIV disease, Ann Pharmacother, doi:10.1345/aph.1A118
Streinu-Cercel, Săndulescu, Preotescu, Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019, Open Forum Infect Dis, doi:10.1093/ofid/ofac053
Tackle Im Tix, None, Cil
Vega, Antila, Perez, SARS-CoV-2-neutralising antibody BGB-DXP593 in mild-to-moderate COVID-19: a multicentre, randomised, double-blind, phase 2 trial, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101832
Vega, None, BGB-DXP
Vega, None, BGB-DXP
Viechtbauer, Conducting meta-analyses in R with the metafor package, J Stat Softw, doi:10.18637/jss.v036.i03
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Wongnak, Schilling, Jittamala, Temporal changes in SARS-CoV-2 clearance kinetics and the optimal design of phase 2 antiviral studies, medRxiv, doi:10.1101/2024.01.16.24301342
DOI record:
{
"DOI": "10.1093/jac/dkae045",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkae045",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Effective antiviral drugs accelerate viral clearance in acute COVID-19 infections; the relationship between accelerating viral clearance and reducing severe clinical outcomes is unclear.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A systematic review was conducted of randomized controlled trials (RCTs) of antiviral therapies in early symptomatic COVID-19, where viral clearance data were available. Treatment benefit was defined clinically as the relative risk of hospitalization/death during follow-up (≥14 days), and virologically as the SARS-CoV-2 viral clearance rate ratio (VCRR). The VCRR is the ratio of viral clearance rates between the intervention and control arms. The relationship between the clinical and virological treatment effects was assessed by mixed-effects meta-regression.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>From 57 potentially eligible RCTs, VCRRs were derived for 44 (52 384 participants); 32 had ≥1 clinical endpoint in each arm. Overall, 9.7% (R2) of the variation in clinical benefit was explained by variation in VCRRs with an estimated linear coefficient of −0.92 (95% CI: −1.99 to 0.13; P = 0.08). However, this estimate was highly sensitive to the inclusion of the recent very large PANORAMIC trial. Omitting this outlier, half the variation in clinical benefit (R2 = 50.4%) was explained by variation in VCRRs [slope −1.47 (95% CI −2.43 to −0.51); P = 0.003], i.e. higher VCRRs were associated with an increased clinical benefit.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Methods of determining viral clearance in COVID-19 studies and the relationship to clinical outcomes vary greatly. As prohibitively large sample sizes are now required to show clinical treatment benefit in antiviral therapeutic assessments, viral clearance is a reasonable surrogate endpoint.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7054-1414",
"affiliation": [
{
"name": "Faculty of Tropical Medicine, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University , Bangkok , Thailand"
}
],
"authenticated-orcid": false,
"family": "Singh",
"given": "Shivani",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0001-8731-5499",
"affiliation": [
{
"name": "Faculty of Tropical Medicine, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University , Bangkok , Thailand"
},
{
"name": "Nuffield Department of Medicine, Centre for Tropical Medicine and Global Health, Oxford University , Oxford , UK"
}
],
"authenticated-orcid": false,
"family": "Boyd",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Tropical Medicine, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University , Bangkok , Thailand"
},
{
"name": "Nuffield Department of Medicine, Centre for Tropical Medicine and Global Health, Oxford University , Oxford , UK"
}
],
"family": "Schilling",
"given": "William H K",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nuffield Department of Medicine, Centre for Tropical Medicine and Global Health, Oxford University , Oxford , UK"
},
{
"name": "Biostatistics Department, Oxford University Clinical Research Unit , 764 Vo Van Kiet, Quan 5 , Ho Chi Minh City, Vietnam"
}
],
"family": "Watson",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Tropical Medicine, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University , Bangkok , Thailand"
},
{
"name": "Nuffield Department of Medicine, Centre for Tropical Medicine and Global Health, Oxford University , Oxford , UK"
}
],
"family": "Mukaka",
"given": "Mavuto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Tropical Medicine, Mahidol Oxford Tropical Medicine Research Unit, Mahidol University , Bangkok , Thailand"
},
{
"name": "Nuffield Department of Medicine, Centre for Tropical Medicine and Global Health, Oxford University , Oxford , UK"
}
],
"family": "White",
"given": "Nicholas J",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T18:29:59Z",
"timestamp": 1708626599000
},
"deposited": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T18:30:27Z",
"timestamp": 1708626627000
},
"funder": [
{
"DOI": "10.13039/100004440",
"award": [
"223195/Z/21/Z)"
],
"doi-asserted-by": "publisher",
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T00:29:39Z",
"timestamp": 1708648179170
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2,
22
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
22
]
],
"date-time": "2024-02-22T00:00:00Z",
"timestamp": 1708560000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkae045/56740824/dkae045.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkae045/56740824/dkae045.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
2,
22
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
22
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1016/j.medj.2021.04.015",
"article-title": "COVID-19: the therapeutic landscape",
"author": "Paules",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Med",
"key": "2024022212394348400_dkae045-B1",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B3",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B4",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1101/2023.06.18.23291566",
"article-title": "Viral clearance as a surrogate of clinical efficacy for COVID-19 therapies in outpatients: a systematic review and meta-analysis",
"author": "Elias",
"doi-asserted-by": "crossref",
"key": "2024022212394348400_dkae045-B6",
"volume-title": "medRxiv",
"year": "2023;"
},
{
"DOI": "10.1093/jac/dkac104",
"article-title": "Clinical relevance of nasopharyngeal SARS-CoV-2 viral load reduction in outpatients with COVID-19",
"author": "Parienti",
"doi-asserted-by": "crossref",
"first-page": "2038",
"journal-title": "J Antimicrob Chemother",
"key": "2024022212394348400_dkae045-B7",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1038/nri2432",
"article-title": "Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases",
"author": "Schneider",
"doi-asserted-by": "crossref",
"first-page": "889",
"journal-title": "Nat Rev Immunol",
"key": "2024022212394348400_dkae045-B8",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1093/cid/cix793",
"article-title": "Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis",
"author": "Natori",
"doi-asserted-by": "crossref",
"first-page": "617",
"journal-title": "Clin Infect Dis",
"key": "2024022212394348400_dkae045-B9",
"volume": "66",
"year": "2018"
},
{
"DOI": "10.1345/aph.1A118",
"article-title": "Viral load as a surrogate end point in HIV disease",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Ann Pharmacother",
"key": "2024022212394348400_dkae045-B10",
"volume": "36",
"year": "2002"
},
{
"DOI": "10.1016/j.antiviral.2019.104553",
"article-title": "Non-invasive biomarkers for chronic hepatitis B virus infection management",
"author": "Charre",
"doi-asserted-by": "crossref",
"first-page": "104553",
"journal-title": "Antivir Res",
"key": "2024022212394348400_dkae045-B11",
"volume": "169",
"year": "2019"
},
{
"DOI": "10.1136/bmjebm-2017-110852",
"article-title": "Assessing the validity of surrogate endpoints in the context of a controversy about the measurement of effectiveness of hepatitis C virus treatment",
"author": "Dobler",
"doi-asserted-by": "crossref",
"first-page": "50",
"journal-title": "BMJ Evid Based Med",
"key": "2024022212394348400_dkae045-B12",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1093/cid/ciab912",
"article-title": "A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "e440",
"journal-title": "Clin Infect Dis",
"key": "2024022212394348400_dkae045-B13",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B14",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"article-title": "SARS-CoV-2 viral load predicts COVID-19 mortality",
"author": "Pujadas",
"doi-asserted-by": "crossref",
"first-page": "e70",
"journal-title": "Lancet Respir Med",
"key": "2024022212394348400_dkae045-B15",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/biostatistics/1.1.49",
"article-title": "The validation of surrogate endpoints in meta-analyses of randomized experiments",
"author": "Buyse",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "Biostatistics",
"key": "2024022212394348400_dkae045-B16",
"volume": "1",
"year": "2000"
},
{
"DOI": "10.1186/2046-4053-4-1",
"article-title": "Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement",
"author": "Moher",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Syst Rev",
"key": "2024022212394348400_dkae045-B17",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1093/infdis/jiad275",
"article-title": "Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: an open-label, randomized controlled adaptive platform trial (PLATCOV)",
"author": "Jittamala",
"doi-asserted-by": "crossref",
"first-page": "1318",
"journal-title": "J Infect Dis",
"key": "2024022212394348400_dkae045-B18",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00493-0",
"article-title": "Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial",
"author": "Schilling",
"doi-asserted-by": "crossref",
"first-page": "36",
"journal-title": "Lancet Infect Dis",
"key": "2024022212394348400_dkae045-B19",
"volume": "24",
"year": "2024"
},
{
"article-title": "Temporal changes in SARS-CoV-2 clearance kinetics and the optimal design of phase 2 antiviral studies",
"author": "Wongnak",
"key": "2024022212394348400_dkae045-B20",
"volume-title": "medRxiv",
"year": "2024"
},
{
"DOI": "10.1186/s13054-017-1609-1",
"article-title": "SOFA and mortality endpoints in randomized controlled trials: a systematic review and meta-regression analysis",
"author": "Grooth",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Crit Care",
"key": "2024022212394348400_dkae045-B21",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.18637/jss.v036.i03",
"article-title": "Conducting meta-analyses in R with the metafor package",
"author": "Viechtbauer",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Stat Softw",
"key": "2024022212394348400_dkae045-B22",
"volume": "36",
"year": "2010"
},
{
"DOI": "10.1016/0197-2456(95)00134-4",
"article-title": "Assessing the quality of reports of randomized clinical trials: is blinding necessary?",
"author": "Jadad",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Control Clin Trials",
"key": "2024022212394348400_dkae045-B23",
"volume": "17",
"year": "1996"
},
{
"author": "Shionogi",
"key": "2024022212394348400_dkae045-B24"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"journal-title": "JAMA",
"key": "2024022212394348400_dkae045-B25",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"article-title": "Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial",
"author": "Montgomery",
"doi-asserted-by": "crossref",
"first-page": "985",
"journal-title": "Lancet Respir Med",
"key": "2024022212394348400_dkae045-B26",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1172/JCI151697",
"article-title": "Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19",
"author": "Ganesh",
"doi-asserted-by": "crossref",
"first-page": "e151697",
"journal-title": "J Clin Investig",
"key": "2024022212394348400_dkae045-B27",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B28",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2023.04.396",
"article-title": "Real-world evaluation of bebtelovimab effectiveness during the period of COVID-19 omicron variants, including BA.4/BA.5",
"author": "Molina",
"doi-asserted-by": "crossref",
"first-page": "34",
"journal-title": "Int J Infect Dis",
"key": "2024022212394348400_dkae045-B29",
"volume": "132",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofac406",
"article-title": "A randomized clinical trial of regdanvimab in high-risk patients with mild-to-moderate coronavirus disease 2019",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "ofac406",
"journal-title": "Open Forum Infect Dis",
"key": "2024022212394348400_dkae045-B30",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.7326/M22-3428",
"article-title": "Safety and efficacy of combination SARS-CoV-2 neutralizing monoclonal antibodies amubarvimab plus romlusevimab in nonhospitalized patients with COVID-19",
"author": "Evering",
"doi-asserted-by": "crossref",
"first-page": "658",
"journal-title": "Ann Intern Med",
"key": "2024022212394348400_dkae045-B31",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2209760",
"article-title": "Early treatment with pegylated interferon lambda for Covid-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "518",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B32",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad088",
"article-title": "Coronavirus disease 2019 convalescent plasma outpatient therapy to prevent outpatient hospitalization: a meta-analysis of individual participant data from 5 randomized trials",
"author": "Levine",
"doi-asserted-by": "crossref",
"first-page": "2077",
"journal-title": "Clin Infect Dis",
"key": "2024022212394348400_dkae045-B33",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1111/cts.13468",
"article-title": "Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: a meta-analysis of individual participant data of randomized trials",
"author": "Mitja",
"doi-asserted-by": "crossref",
"first-page": "524",
"journal-title": "Clin Transl Sci",
"key": "2024022212394348400_dkae045-B34",
"volume": "16",
"year": "2023"
},
{
"article-title": "Ivermectin for preventing and treating COVID-19",
"author": "Popp",
"first-page": "CD015017",
"journal-title": "Cochrane Database Syst Rev",
"key": "2024022212394348400_dkae045-B35",
"volume": "6",
"year": "2022"
},
{
"article-title": "Efficacy and safety of favipiravir in treating COVID-19 patients: a meta-analysis of randomized control trials",
"author": "Batool",
"first-page": "e33676",
"journal-title": "Cureus",
"key": "2024022212394348400_dkae045-B36",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2022.12.028",
"article-title": "Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial",
"author": "Kaizer",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Int J Infect Dis",
"key": "2024022212394348400_dkae045-B37",
"volume": "128",
"year": "2023"
},
{
"DOI": "10.1007/s00228-022-03380-5",
"article-title": "Efficacy and safety of nitazoxanide in treating SARS-CoV-2 infection: a systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials",
"author": "Martins-Filho",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "Eur J Clin Pharmacol",
"key": "2024022212394348400_dkae045-B38",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.10.012",
"article-title": "Efficacy and safety of fluvoxamine for the treatment of COVID-19 patients: a systematic review and meta-analysis",
"author": "Cheema",
"doi-asserted-by": "crossref",
"first-page": "702",
"journal-title": "J Infect",
"key": "2024022212394348400_dkae045-B39",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2021.100993",
"article-title": "Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial",
"author": "Parienti",
"doi-asserted-by": "crossref",
"first-page": "100993",
"journal-title": "EClinicalMedicine",
"key": "2024022212394348400_dkae045-B40",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2022.104322",
"article-title": "Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: a randomised, open-label, multi-arm, phase 2 clinical trial",
"author": "Chandiwana",
"doi-asserted-by": "crossref",
"first-page": "104322",
"journal-title": "eBioMedicine",
"key": "2024022212394348400_dkae045-B41",
"volume": "86",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkaa501",
"article-title": "Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial",
"author": "Roozbeh",
"doi-asserted-by": "crossref",
"first-page": "753",
"journal-title": "J Antimicrob Chemother",
"key": "2024022212394348400_dkae045-B42",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.2337/dc22-2539",
"article-title": "Metformin for treatment of acute COVID-19: systematic review of clinical trial data against SARS-CoV-2",
"author": "Erickson",
"doi-asserted-by": "crossref",
"first-page": "1432",
"journal-title": "Diabetes Care",
"key": "2024022212394348400_dkae045-B43",
"volume": "46",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "2024022212394348400_dkae045-B44",
"volume": "401",
"year": "2023"
},
{
"article-title": "Efficacy and safety of molnupiravir in mild COVID-19 patients in India",
"author": "Sinha",
"first-page": "e31508",
"journal-title": "Cureus",
"key": "2024022212394348400_dkae045-B45",
"volume": "14",
"year": "2022"
},
{
"article-title": "Detection of influential observation in linear regression",
"author": "Cook",
"first-page": "15",
"journal-title": "Technometrics",
"key": "2024022212394348400_dkae045-B46",
"volume": "19",
"year": "1977"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "Fischer",
"doi-asserted-by": "crossref",
"first-page": "eabl7430",
"journal-title": "Sci Transl Med",
"key": "2024022212394348400_dkae045-B47",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2024022212394348400_dkae045-B48",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac053",
"article-title": "Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019",
"author": "Streinu-Cercel",
"doi-asserted-by": "crossref",
"first-page": "ofac053",
"journal-title": "Open Forum Infect Dis",
"key": "2024022212394348400_dkae045-B49",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciaa1009",
"article-title": "Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial",
"author": "Mitjà",
"doi-asserted-by": "crossref",
"first-page": "e4073",
"journal-title": "Clin Infect Dis",
"key": "2024022212394348400_dkae045-B50",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00545-2",
"article-title": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial",
"author": "Alemany",
"doi-asserted-by": "crossref",
"first-page": "278",
"journal-title": "Lancet Respir Med",
"key": "2024022212394348400_dkae045-B51",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-32551-2",
"article-title": "Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19",
"author": "Chew",
"doi-asserted-by": "crossref",
"first-page": "4931",
"journal-title": "Nat Commun",
"key": "2024022212394348400_dkae045-B52",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1101/2022.03.10.22272100",
"article-title": "Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"key": "2024022212394348400_dkae045-B53",
"volume-title": "medRxiv",
"year": "2022;"
},
{
"DOI": "10.1016/S2213-2600(20)30566-X",
"article-title": "Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial",
"author": "Feld",
"doi-asserted-by": "crossref",
"first-page": "498",
"journal-title": "Lancet Respir Med",
"key": "2024022212394348400_dkae045-B54",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2022.08.005",
"article-title": "Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "208",
"journal-title": "Clin Infect Dis",
"key": "2024022212394348400_dkae045-B55",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2022.101703",
"article-title": "Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial",
"author": "McMahon",
"doi-asserted-by": "crossref",
"first-page": "101703",
"journal-title": "EClinicalMedicine",
"key": "2024022212394348400_dkae045-B56",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2023.10039",
"article-title": "Safety and efficacy of combined tixagevimab and cilgavimab administered intramuscularly or intravenously in nonhospitalized patients with COVID-19: 2 randomized clinical trials",
"author": "Ignacio",
"doi-asserted-by": "crossref",
"first-page": "e2310039",
"journal-title": "JAMA Netw Open",
"key": "2024022212394348400_dkae045-B57",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.101832",
"article-title": "SARS-CoV-2-neutralising antibody BGB-DXP593 in mild-to-moderate COVID-19: a multicentre, randomised, double-blind, phase 2 trial",
"author": "Vega",
"doi-asserted-by": "crossref",
"first-page": "101832",
"journal-title": "EClinicalMedicine",
"key": "2024022212394348400_dkae045-B58",
"volume": "57",
"year": "2023"
},
{
"DOI": "10.1038/s41467-021-22177-1",
"article-title": "Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial",
"author": "Jagannathan",
"doi-asserted-by": "crossref",
"first-page": "1967",
"journal-title": "Nat Commun",
"key": "2024022212394348400_dkae045-B59",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"article-title": "A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "101310",
"journal-title": "EClinicalMedicine",
"key": "2024022212394348400_dkae045-B60",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2022.07.003",
"article-title": "The effect of ivermectin on the viral load and culture viability in early treatment of nonhospitalized patients with mild COVID-19–a double-blind, randomized placebo-controlled trial",
"author": "Biber",
"doi-asserted-by": "crossref",
"first-page": "733",
"journal-title": "Int J Infect Dis",
"key": "2024022212394348400_dkae045-B61",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "2024022212394348400_dkae045-B62",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1183/13993003.03725-2020",
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial",
"author": "Rocco",
"doi-asserted-by": "crossref",
"first-page": "2003725",
"journal-title": "Eur Respir J",
"key": "2024022212394348400_dkae045-B63",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1186/s12879-023-08835-3",
"article-title": "Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial",
"author": "Luvira",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "BMC Infect Dis",
"key": "2024022212394348400_dkae045-B64",
"volume": "24",
"year": "2024"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkae045/7612573"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "The relationship between viral clearance rates and disease progression in early symptomatic COVID-19: a systematic review and meta-regression analysis",
"type": "journal-article"
}