Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(20)30566-X, ILIAD Cohort A, NCT04354259, Nov 2020 (preprint)
Small outpatient RCT with 30 peginterferon lambda and 30 control patients, showing improved viral clearance with treatment. Single subcutaneous injection of peginterferon lambda 180μg.
|
risk of hospitalization, no change, RR 1.00, p = 1.00, treatment 1 of 30 (3.3%), control 1 of 30 (3.3%).
|
|
risk of ER visit, 75.0% lower, RR 0.25, p = 0.35, treatment 1 of 30 (3.3%), control 4 of 30 (13.3%), NNT 10.0.
|
|
risk of no viral clearance, 66.4% lower, RR 0.34, p = 0.03, treatment 6 of 30 (20.0%), control 11 of 30 (36.7%), NNT 6.0, inverted to make RR<1 favor treatment, odds ratio converted to relative risk, adjusted for baseline viral load, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Feld et al., 12 Nov 2020, Double Blind Randomized Controlled Trial, placebo-controlled, Canada, peer-reviewed, 35 authors, study period 18 May, 2020 - 4 September, 2020, average treatment delay 4.3 days, trial NCT04354259 (history) (ILIAD Cohort A).
Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial
doi:10.1016/S2213-2600
Background To date, only monoclonal antibodies have been shown to be effective for outpatients with COVID-19. Interferon lambda-1 is a type III interferon involved in innate antiviral responses with activity against respiratory pathogens. We aimed to investigate the safety and efficacy of peginterferon lambda in the treatment of outpatients with mild-to-moderate COVID-19.
Methods In this double-blind, placebo-controlled trial, outpatients with laboratory-confirmed COVID-19 were randomly assigned to a single subcutaneous injection of peginterferon lambda 180 µg or placebo within 7 days of symptom onset or first positive swab if asymptomatic. Participants were randomly assigned (1:1) using a computergenerated randomisation list created with a randomisation schedule in blocks of four. At the time of administration, study nurses received a sealed opaque envelope with the treatment allocation number. The primary endpoint was the proportion of patients who were negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA on day 7 after the injection, analysed by a χ² test following an intention-to-treat principle. Prespecified analysis of the primary endpoint, adjusted for baseline viral load, using bivariate logistic regression was done. The trial is now complete. This trial is registered with ClinicalTrials.gov, NCT04354259.
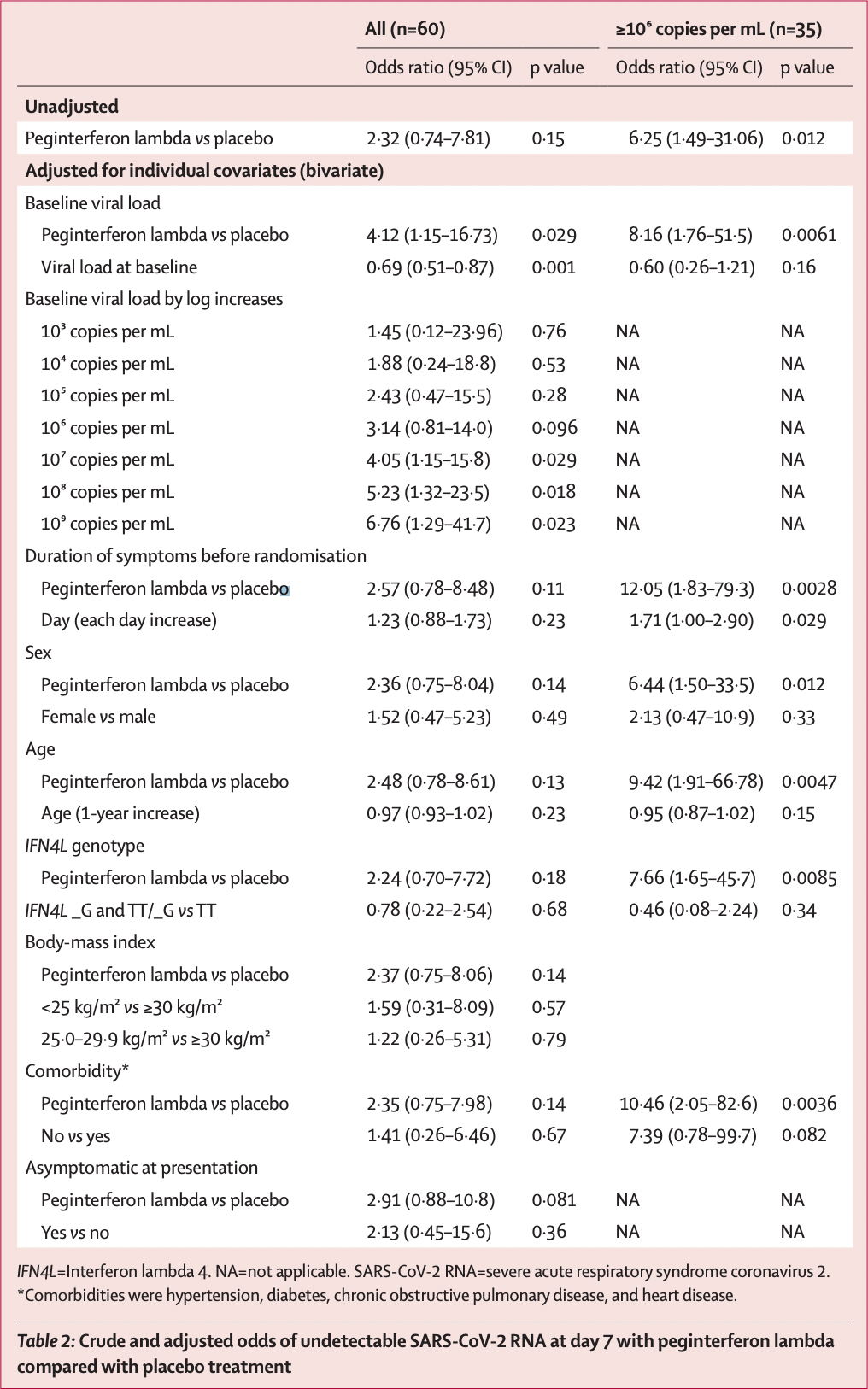
Findings Between May 18, and Sept 4, 2020, we recruited 30 patients per group. The decline in SARS-CoV-2 RNA was greater in those treated with peginterferon lambda than placebo from day 3 onwards, with a difference of 2•42 log copies per mL at day 7 (p=0•0041). By day 7, 24 (80%) participants in the peginterferon lambda group had an undetectable viral load, compared with 19 (63%) in the placebo group (p=0•15). After controlling for baseline viral load, patients in the peginterferon lambda group were more likely to have undetectable virus by day 7 than were those in the placebo group (odds ratio [OR] 4•12 [95% CI 1•15-16•73; p=0•029). Of those with baseline viral load above 10⁶ copies per mL, 15 (79%) of 19 patients in the peginterferon lambda group had undetectable virus on day 7, compared with six (38%) of 16 in the placebo group (OR 6•25 [95% CI 1•49-31•06]; p=0•012). Peginterferon lambda was well tolerated, and adverse events were similar between groups with mild and transient aminotransferase, concentration increases more frequently observed in the peginterferon lambda group. Two individuals met the threshold of grade 3 increase, one in each group, and no other grade 3 or 4 laboratory adverse events were reported. Interpretation Peginterferon lambda accelerated viral decline in outpatients with COVID-19, increasing the proportion of patients with viral clearance by day 7, particularly in those with high baseline viral load. Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding.
References
Aoki, Macleod, Paggiaro, Early administration of oral oseltamivir increases the benefits of influenza treatment, J Antimicrob Chemother
Bastard, Rosen, Zhang, Autoantibodies against type I IFNs in patients with life-threatening COVID-19, Science
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-final report, N Engl J Med
Bullard, Dust, Funk, Predicting infectious SARS-CoV-2 from diagnostic samples, Clin Infect Dis
Carrat, Duval, Tubach, Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households, Antivir Ther
Chan, Ahn, Chang, Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: a randomized phase 2b study (LIRA-B), J Hepatol
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N Engl J Med
Chu, Chan, Wang, Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19, Clin Infect Dis
Davidson, Mccabe, Crotta, IFNλ is a potent antiinfluenza therapeutic without the inflammatory side effects of IFNα treatment, EMBO Mol Med
Dinnon Kh 3rd, Leist, Schäfer, A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures, Nature
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Ge, Fellay, Thompson, Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance, Nature
Hadjadj, Yatim, Barnabei, Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19-preliminary report, N Engl J Med, doi:10.1056/NEJMoa2021436
Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Jagannathan, Andrews, Bonilla, Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial, medRxiv, doi:10.1101/2020.11.18.20234161
Luo, Cassidy, Jeffers, Reddy, Bruno et al., Interferon-stimulated gene expression in black and white hepatitis C patients during peginterferon alfa-2a combination therapy, Clin Gastroenterol Hepatol
Monk, Marsden, Tear, Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial, Lancet Respir Med
Muir, Arora, Everson, A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection, J Hepatol
Naymagon, Zubizarreta, Feld, Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19, Thromb Res
Pan, Peto, Quarraisha, Repurposed antiviral drugs for COVID-19-interim WHO Solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Park, Iwasaki, Type I and type III interferons-induction, signaling, evasion, and application to combat COVID-19, Cell Host Microbe
Prokunina-Olsson, Dickenson, COVID-19 and emerging viral infections: the case for interferon lambda, J Exp Med
Prokunina-Olsson, Muchmore, Tang, A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus, Nat Genet
Pujadas, Chaudhry, Mcbride, SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir Med
Reddy, Hoofnagle, Tong, Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group, Hepatology
Scohy, Anantharajah, Bodéus, Kabamba-Mukadi, Verroken et al., Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis, J Clin Virol
Scola, Bideau, Andreani, Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards, Eur J Clin Microbiol Infect Dis
Song, Zhang, Qian, Li, Shen et al., A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19)
Syedbasha, Egli, Weinreich, Sivapalasingam, Norton, Interferon lambda: modulating immunity in infectious diseases
Thomas, Thio, Martin, Genetic variation in IL28B and spontaneous clearance of hepatitis C virus, Nature
Vanderheiden, Ralfs, Chirkova, Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures, J Virol
Vidali, Morosetti, Cossu, D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review, ERJ Open Res
Xie, Jiang, Zeng, Liu, Combination antiviral therapy with lopinavir/ritonavir, arbidol and interferon-α1b for COVID-19
Zhang, Bastard, Liu, Inborn errors of type I IFN immunity in patients with life-threatening COVID-19, Science
Zheng, Yu, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study, BMJ
Zhou, Chen, Shannon, Interferon-α2b treatment for COVID-19, Front Immunol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/s2213-2600(20)30566-x",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/s2213-2600(20)30566-x",
"alternative-id": [
"S221326002030566X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(20)30566-X"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Feld",
"given": "Jordan J",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kandel",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biondi",
"given": "Mia J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kozak",
"given": "Robert A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zahoor",
"given": "Muhammad Atif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lemieux",
"given": "Camille",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Borgia",
"given": "Sergio M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boggild",
"given": "Andrea K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Powis",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCready",
"given": "Janine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tan",
"given": "Darrell H S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Tiffany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coburn",
"given": "Bryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Deepali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Humar",
"given": "Atul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Adrienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O'Neil",
"given": "Braden",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noureldin",
"given": "Seham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Booth",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smookler",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aleyadeh",
"given": "Wesam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Anjali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barber",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casey",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiebert",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mistry",
"given": "Henna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choong",
"given": "Ingrid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hislop",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santer",
"given": "Deanna M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorne Tyrrell",
"given": "D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Glenn",
"given": "Jeffrey S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gehring",
"given": "Adam J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Janssen",
"given": "Harry L A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hansen",
"given": "Bettina E",
"sequence": "additional"
}
],
"container-title": [
"The Lancet Respiratory Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
7
]
],
"date-time": "2021-02-07T06:54:31Z",
"timestamp": 1612680871000
},
"deposited": {
"date-parts": [
[
2021,
5,
10
]
],
"date-time": "2021-05-10T14:31:15Z",
"timestamp": 1620657075000
},
"indexed": {
"date-parts": [
[
2022,
2,
17
]
],
"date-time": "2022-02-17T12:07:38Z",
"timestamp": 1645099658720
},
"is-referenced-by-count": 65,
"issn-type": [
{
"type": "print",
"value": "2213-2600"
}
],
"issue": "5",
"issued": {
"date-parts": [
[
2021,
5
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
1
]
],
"date-time": "2021-05-01T00:00:00Z",
"timestamp": 1619827200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S221326002030566X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S221326002030566X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "498-510",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
5
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(20)30566-X_bib1",
"volume": "383",
"year": "2020"
},
{
"article-title": "Dexamethasone in hospitalized patients with COVID-19—preliminary report",
"author": "Horby",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(20)30566-X_bib2",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19",
"author": "Chen",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(20)30566-X_bib3",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkg007",
"article-title": "Early administration of oral oseltamivir increases the benefits of influenza treatment",
"author": "Aoki",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S2213-2600(20)30566-X_bib4",
"volume": "51",
"year": "2003"
},
{
"DOI": "10.3851/IMP2128",
"article-title": "Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households",
"author": "Carrat",
"doi-asserted-by": "crossref",
"first-page": "1085",
"journal-title": "Antivir Ther",
"key": "10.1016/S2213-2600(20)30566-X_bib5",
"volume": "17",
"year": "2012"
},
{
"DOI": "10.1016/j.chom.2020.05.008",
"article-title": "Type I and type III interferons—induction, signaling, evasion, and application to combat COVID-19",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "870",
"journal-title": "Cell Host Microbe",
"key": "10.1016/S2213-2600(20)30566-X_bib6",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1084/jem.20200653",
"article-title": "COVID-19 and emerging viral infections: the case for interferon lambda",
"author": "Prokunina-Olsson",
"doi-asserted-by": "crossref",
"journal-title": "J Exp Med",
"key": "10.1016/S2213-2600(20)30566-X_bib7",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.15252/emmm.201606413",
"article-title": "IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment",
"author": "Davidson",
"doi-asserted-by": "crossref",
"first-page": "1099",
"journal-title": "EMBO Mol Med",
"key": "10.1016/S2213-2600(20)30566-X_bib8",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1038/s41586-020-2708-8",
"article-title": "A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures",
"author": "Dinnon",
"doi-asserted-by": "crossref",
"first-page": "560",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(20)30566-X_bib9",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00985-20",
"article-title": "Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures",
"author": "Vanderheiden",
"doi-asserted-by": "crossref",
"first-page": "e00985",
"journal-title": "J Virol",
"key": "10.1016/S2213-2600(20)30566-X_bib10",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1016/j.jhep.2014.07.022",
"article-title": "A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection",
"author": "Muir",
"doi-asserted-by": "crossref",
"first-page": "1238",
"journal-title": "J Hepatol",
"key": "10.1016/S2213-2600(20)30566-X_bib11",
"volume": "61",
"year": "2014"
},
{
"DOI": "10.1016/j.jhep.2015.12.018",
"article-title": "Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: a randomized phase 2b study (LIRA-B)",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "1011",
"journal-title": "J Hepatol",
"key": "10.1016/S2213-2600(20)30566-X_bib12",
"volume": "64",
"year": "2016"
},
{
"DOI": "10.1038/ng.2521",
"article-title": "A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus",
"author": "Prokunina-Olsson",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "Nat Genet",
"key": "10.1016/S2213-2600(20)30566-X_bib13",
"volume": "45",
"year": "2013"
},
{
"DOI": "10.1038/nature08309",
"article-title": "Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance",
"author": "Ge",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(20)30566-X_bib14",
"volume": "461",
"year": "2009"
},
{
"DOI": "10.1038/nature08463",
"article-title": "Genetic variation in IL28B and spontaneous clearance of hepatitis C virus",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "798",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(20)30566-X_bib15",
"volume": "461",
"year": "2009"
},
{
"article-title": "A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19)",
"author": "Song",
"journal-title": "J Zhejiang University",
"key": "10.1016/S2213-2600(20)30566-X_bib16",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/S2213-2600(20)30566-X_bib17",
"volume": "56",
"year": "2020"
},
{
"article-title": "Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study",
"author": "Zheng",
"journal-title": "BMJ",
"key": "10.1016/S2213-2600(20)30566-X_bib18",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"article-title": "SARS-CoV-2 viral load predicts COVID-19 mortality",
"author": "Pujadas",
"doi-asserted-by": "crossref",
"first-page": "e70",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(20)30566-X_bib19",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa638",
"article-title": "Predicting infectious SARS-CoV-2 from diagnostic samples",
"author": "Bullard",
"doi-asserted-by": "crossref",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2213-2600(20)30566-X_bib20",
"year": "2020"
},
{
"DOI": "10.1007/s10096-020-03913-9",
"article-title": "Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards",
"author": "La Scola",
"doi-asserted-by": "crossref",
"first-page": "1059",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "10.1016/S2213-2600(20)30566-X_bib21",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2017.00119",
"article-title": "Interferon lambda: modulating immunity in infectious diseases",
"author": "Syedbasha",
"doi-asserted-by": "crossref",
"first-page": "119",
"journal-title": "Front Immunol",
"key": "10.1016/S2213-2600(20)30566-X_bib22",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(20)30566-X_bib23",
"volume": "384",
"year": "2021"
},
{
"article-title": "Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial",
"author": "Jagannathan",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(20)30566-X_bib24",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa410",
"article-title": "Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19",
"author": "Chu",
"doi-asserted-by": "crossref",
"first-page": "1400",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2213-2600(20)30566-X_bib25",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6027",
"article-title": "Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients",
"author": "Hadjadj",
"doi-asserted-by": "crossref",
"first-page": "718",
"journal-title": "Science",
"key": "10.1016/S2213-2600(20)30566-X_bib26",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4585",
"article-title": "Autoantibodies against type I IFNs in patients with life-threatening COVID-19",
"author": "Bastard",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/S2213-2600(20)30566-X_bib27",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4570",
"article-title": "Inborn errors of type I IFN immunity in patients with life-threatening COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"journal-title": "Science",
"key": "10.1016/S2213-2600(20)30566-X_bib28",
"volume": "370",
"year": "2020"
},
{
"article-title": "Interferon-α2b treatment for COVID-19",
"author": "Zhou",
"journal-title": "Front Immunol",
"key": "10.1016/S2213-2600(20)30566-X_bib29",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3851/IMP3362",
"article-title": "Combination antiviral therapy with lopinavir/ritonavir, arbidol and interferon-α1b for COVID-19",
"author": "Xie",
"doi-asserted-by": "crossref",
"journal-title": "Antivir Ther",
"key": "10.1016/S2213-2600(20)30566-X_bib30",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(20)30566-X_bib31",
"volume": "395",
"year": "2020"
},
{
"article-title": "Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results",
"author": "Pan",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(20)30566-X_bib32",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30511-7",
"article-title": "Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial",
"author": "Monk",
"doi-asserted-by": "crossref",
"first-page": "196",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(20)30566-X_bib33",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(20)30566-X_bib34",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104455",
"article-title": "Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis",
"author": "Scohy",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Virol",
"key": "10.1016/S2213-2600(20)30566-X_bib35",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.1002/hep.510300319",
"article-title": "Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group",
"author": "Reddy",
"doi-asserted-by": "crossref",
"first-page": "787",
"journal-title": "Hepatology",
"key": "10.1016/S2213-2600(20)30566-X_bib36",
"volume": "30",
"year": "1999"
},
{
"DOI": "10.1016/S1542-3565(04)00615-9",
"article-title": "Interferon-stimulated gene expression in black and white hepatitis C patients during peginterferon alfa-2a combination therapy",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "10.1016/S2213-2600(20)30566-X_bib37",
"volume": "3",
"year": "2005"
},
{
"DOI": "10.1183/23120541.00260-2020",
"article-title": "D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review",
"author": "Vidali",
"doi-asserted-by": "crossref",
"first-page": "00260",
"journal-title": "ERJ Open Res",
"key": "10.1016/S2213-2600(20)30566-X_bib38",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.08.032",
"article-title": "Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19",
"author": "Naymagon",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Thromb Res",
"key": "10.1016/S2213-2600(20)30566-X_bib39",
"volume": "196",
"year": "2020"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"score": 1,
"short-container-title": [
"The Lancet Respiratory Medicine"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": [
"Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}