A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac406, NCT04602000, Aug 2022
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 1,315 outpatients in South Korea, showing lower progression and improved recovery with regdanvimab.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
|
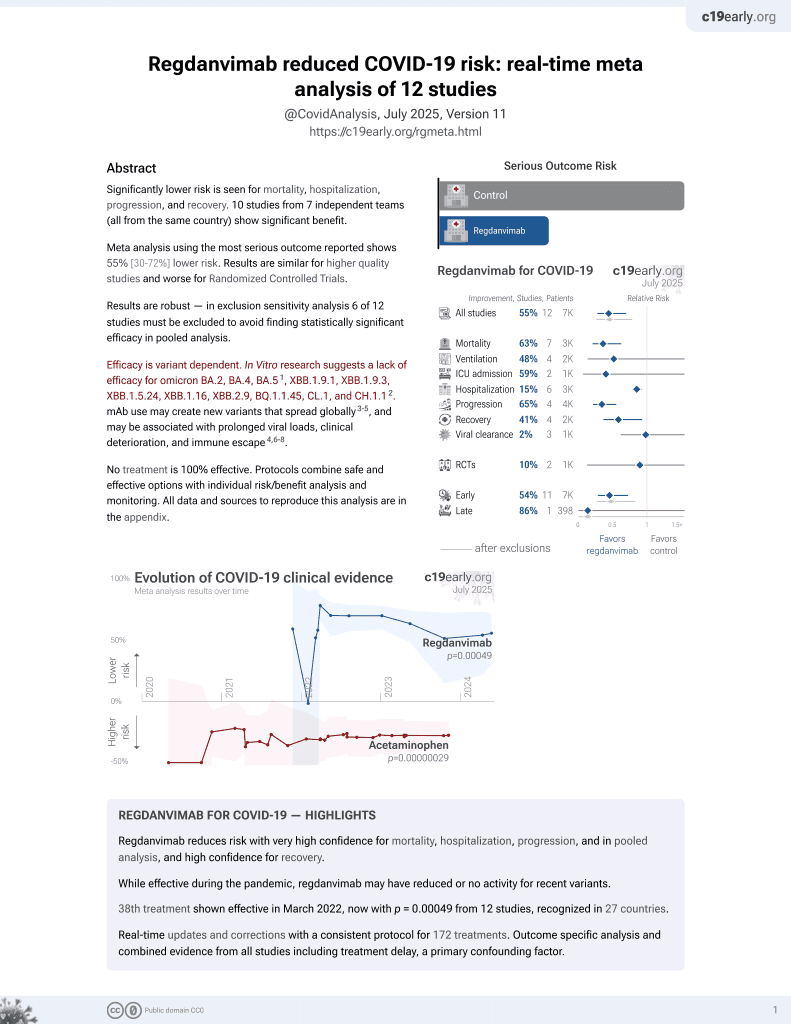
risk of death, 49.8% lower, RR 0.50, p = 1.00, treatment 1 of 656 (0.2%), control 2 of 659 (0.3%), NNT 662.
|
|
risk of mechanical ventilation, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 656 (0.0%), control 3 of 659 (0.5%), NNT 220, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 90.9% lower, RR 0.09, p = 0.06, treatment 0 of 656 (0.0%), control 5 of 659 (0.8%), NNT 132, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 69.2% lower, RR 0.31, p < 0.001, treatment 15 of 656 (2.3%), control 49 of 659 (7.4%), NNT 19.
|
|
risk of hospitalization, 69.1% lower, RR 0.31, p < 0.001, treatment 16 of 656 (2.4%), control 52 of 659 (7.9%), NNT 18.
|
|
risk of progression, 69.7% lower, RR 0.30, p < 0.001, treatment 16 of 656 (2.4%), control 53 of 659 (8.0%), NNT 18.
|
|
risk of progression, 71.6% lower, RR 0.28, p < 0.001, treatment 14 of 446 (3.1%), control 48 of 434 (11.1%), NNT 13, high-risk patients.
|
|
risk of no viral clearance, 32.4% lower, RR 0.68, p < 0.001, treatment 612, control 618, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kim et al., 8 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, median age 48.0, 24 authors, study period 18 January, 2021 - 24 April, 2021, average treatment delay 4.0 days, trial NCT04602000 (history).
Contact: helppl@gilhospital.com.
A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019
Open Forum Infectious Diseases, doi:10.1093/ofid/ofac406
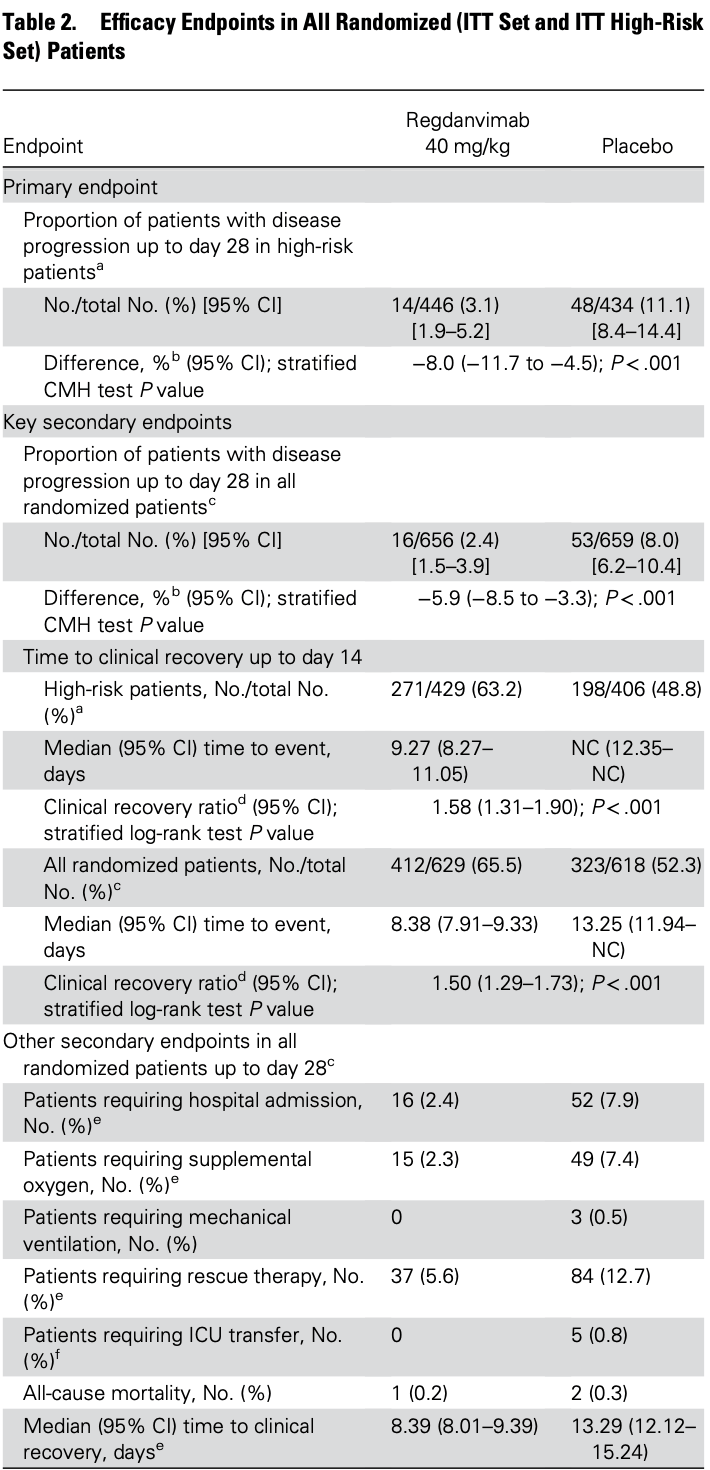
Background. We evaluated clinical effectiveness of regdanvimab (CT-P59), a severe acute respiratory syndrome coronavirus 2 neutralizing monoclonal antibody, in reducing disease progression and clinical recovery time in patients with mild-to-moderate coronavirus disease 2019 (COVID-19), primarily Alpha variant. Methods. This was phase 3 of a phase 2/3 parallel-group, double-blind, randomized clinical trial. Outpatients with mild-tomoderate COVID-19 were randomized to single-dose regdanvimab 40 mg/kg (n = 656) or placebo (n = 659), alongside standard of care. The primary endpoint was COVID-19 disease progression up to day 28 among "high-risk" patients. Key secondary endpoints were disease progression (all randomized patients) and time to recovery (high-risk and all randomized patients). Results. Of 1315 randomized patients, 880 were high risk; the majority were infected with Alpha variant. The proportion with disease progression was lower (14/446, 3.1% [95% confidence interval {CI}, 1.9%-5.2%] vs 48/434, 11.1% [95% CI, 8.4%-14.4%]; P < .001) and time to recovery was shorter (median, 9.27 days [95% CI, 8.27-11.05 days] vs not reached [95% CI, 12.35-not calculable]; P < .001) with regdanvimab than placebo. Consistent improvements were seen in all randomized and non-high-risk patients who received regdanvimab. Viral load reductions were more rapid with regdanvimab. Infusion-related reactions occurred in 11 patients (4/652 [0.6%] regdanvimab, 7/650 [1.1%] placebo). Treatment-emergent serious adverse events were reported in 5 of (4/652 [0.6%] regdanvimab and 1/650 [0.2%] placebo). Conclusions. Regdanvimab was an effective treatment for patients with mild-to-moderate COVID-19, significantly reducing disease progression and clinical recovery time without notable safety concerns prior to the emergence of the Omicron variant. Clinical Trials Registration. NCT04602000; 2020-003369-20 (EudraCT).
Supplementary Data Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
Auvigne, Vaux, Strat, Severe hospital events following symptomatic infection with SARS-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study, EClinicalMedicine
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Davies, Kassanjee, Rousseau, Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa, Trop Med Int Health
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med
Ferguson, Ghani, Hinsley, Volz, Hospitalisation risk for Omicron cases in England
Gisaid, Tracking of variants: VOC Delta, relative variant genome frequency per region
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Hoffmann, Corleis, Rauch, CVnCov and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2, Nat Commun
Ju, Zheng, Guo, Molecular basis of broad neutralization against SARS-CoV-2 variants including Omicron by a human antibody
Kim, Jang, Hong, Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection, Clin Ther
Kim, Ryu, Lee, A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun
Lee, Lee, Ko, Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease, Front Immunol
Mancuso, Venturelli, Vicentini, Temporal profile and determinants of viral shedding and of viral clearance confirmation on nasopharyngeal swabs from SARS-CoV-2-positive subjects: a population-based prospective cohort study in Reggio Emilia, Italy, BMJ Open
Marston, Paules, Fauci, Monoclonal antibodies for emerging infectious diseases-borrowing from history, N Engl J Med
Mohammad, Aziz, Mahri, Obesity and COVID-19: what makes obese host so vulnerable?, Immun Ageing
Naz, Patel, Rifai, Turlington, Shapiro, previously infected with COVID-19
Perkin, Heap, Crerar-Gilbert, Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: a hospital case note review, BMJ Open
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature
Pollard, Morran, Al, The COVID-19 pandemic: a global health crisis, Physiol Genomics
Rodriguez-Diaz, Guilamo-Ramos, Mena, Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics, Ann Epidemiol
Ryu, Kang, Noh, The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2, Biochem Biophys Res Commun
Ryu, Song, Kim, Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant, Biochem Biophys Res Commun
Schulz, Hoffmann, Roth, Augmented neutralization of SARS-CoV-2 Omicron variant by boost vaccination and monoclonal antibodies, Eur J Immunol
Sheikh, Kerr, Woolhouse, Mcmenamin, Robertson, Severity of Omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested testnegative design, Lancet Infect Dis
Streinu-Cercel, Săndulescu, Preotescu, Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019, Open Forum Infect Dis
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat Rev Immunol
Varikasuvu, Dutt, Thangappazham, Varshney, Diabetes and COVID-19: a pooled analysis related to disease severity and mortality, Prim Care Diabetes
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Wiley, Kubes, Cobb, Age, comorbid conditions, and racial disparities in COVID-19 outcomes, J Racial Ethn Health Disparities
Wire, Celltrion's monoclonal antibody treatment for COVID-19, regdanvimab
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study, Lancet
Yamasoba, Kimura, Kosugi, Neutralization sensitivity of Omicron BA.2.75 to therapeutic monoclonal antibodies, doi:10.1101/2022.07.14.500041
Zhou, Chi, Lv, Wang, Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19), Diabetes Metab Res Rev
DOI record:
{
"DOI": "10.1093/ofid/ofac406",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofac406",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>We evaluated clinical effectiveness of regdanvimab (CT-P59), a severe acute respiratory syndrome coronavirus 2 neutralizing monoclonal antibody, in reducing disease progression and clinical recovery time in patients with mild-to-moderate coronavirus disease 2019 (COVID-19), primarily Alpha variant.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was phase 3 of a phase 2/3 parallel-group, double-blind, randomized clinical trial. Outpatients with mild-to-moderate COVID-19 were randomized to single-dose regdanvimab 40 mg/kg (n = 656) or placebo (n = 659), alongside standard of care. The primary endpoint was COVID-19 disease progression up to day 28 among “high-risk” patients. Key secondary endpoints were disease progression (all randomized patients) and time to recovery (high-risk and all randomized patients).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of 1315 randomized patients, 880 were high risk; the majority were infected with Alpha variant. The proportion with disease progression was lower (14/446, 3.1% [95% confidence interval {CI}, 1.9%–5.2%] vs 48/434, 11.1% [95% CI, 8.4%–14.4%]; P &lt; .001) and time to recovery was shorter (median, 9.27 days [95% CI, 8.27–11.05 days] vs not reached [95% CI, 12.35–not calculable]; P &lt; .001) with regdanvimab than placebo. Consistent improvements were seen in all randomized and non-high-risk patients who received regdanvimab. Viral load reductions were more rapid with regdanvimab. Infusion-related reactions occurred in 11 patients (4/652 [0.6%] regdanvimab, 7/650 [1.1%] placebo). Treatment-emergent serious adverse events were reported in 5 of (4/652 [0.6%] regdanvimab and 1/650 [0.2%] placebo).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Regdanvimab was an effective treatment for patients with mild-to-moderate COVID-19, significantly reducing disease progression and clinical recovery time without notable safety concerns prior to the emergence of the Omicron variant.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>NCT04602000; 2020-003369-20 (EudraCT).</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Internal Medicine, Division of Infectious Diseases, Incheon Medical Center , Incheon , Republic of Korea"
}
],
"family": "Kim",
"given": "Jin Yong",
"sequence": "first"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof Dr Matei Balş,” Carol Davila University of Medicine and Pharmacy , Bucharest , Romania"
}
],
"family": "Săndulescu",
"given": "Oana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof Dr Matei Balş,” Carol Davila University of Medicine and Pharmacy , Bucharest , Romania"
}
],
"family": "Preotescu",
"given": "Liliana-Lucia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Oaxaca Site Management Organization , Oaxaca , Mexico"
}
],
"family": "Rivera-Martínez",
"given": "Norma E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "City Clinical Hospital 12 , Kyiv , Ukraine"
},
{
"name": "ARENSIA Exploratory Medicine , Kyiv , Ukraine"
}
],
"family": "Dobryanska",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Lucian Blaga University of Sibiu, Emergency Clinical County Hospital , Sibiu , Romania"
}
],
"family": "Birlutiu",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Hospital of Infectious Diseases “Sfanta Parascheva,” University of Medicine and Pharmacy “Gr. T. Popa,” Iasi , Romania"
}
],
"family": "Miftode",
"given": "Egidia G",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institutul Oncologic din Republica Moldova Republican Clinical Hospital “T. Moşneaga,” ARENSIA Exploratory Medicine , Chisinau , Moldova"
}
],
"family": "Gaibu",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stefan cel Mare University , Suceava , Romania"
}
],
"family": "Caliman-Sturdza",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dr Victor Babes Clinical Hospital for Tropical and Infectious Diseases , Bucharest , Romania"
}
],
"family": "Florescu",
"given": "Simin-Aysel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Division of Infectious Diseases, Gil Medical Center, Gachon University College of Medicine , Incheon , Republic of Korea"
}
],
"family": "Shi",
"given": "Hye Jin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof Dr Matei Balş,” Carol Davila University of Medicine and Pharmacy , Bucharest , Romania"
}
],
"family": "Streinu-Cercel",
"given": "Anca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof Dr Matei Balş,” Carol Davila University of Medicine and Pharmacy , Bucharest , Romania"
}
],
"family": "Streinu-Cercel",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Lee",
"given": "Sang Joon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Kim",
"given": "Sung Hyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Chang",
"given": "Ilsung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Bae",
"given": "Yun Ju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Suh",
"given": "Jee Hye",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Chung",
"given": "Da Rae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Kim",
"given": "Sun Jung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Kim",
"given": "Mi Rim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Lee",
"given": "Seul Gi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc , Incheon , Republic of Korea"
}
],
"family": "Park",
"given": "Gahee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Division of Infectious Diseases, Gil Medical Center, Gachon University College of Medicine , Incheon , Republic of Korea"
}
],
"family": "Eom",
"given": "Joong Sik",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T01:17:55Z",
"timestamp": 1659921475000
},
"deposited": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T16:51:19Z",
"timestamp": 1661791879000
},
"funder": [
{
"name": "Celltrion, Inc"
},
{
"name": "Korea Health Technology R&D Project"
},
{
"name": "the Korea Health Industry Development Institute"
},
{
"award": [
"HQ20C0073"
],
"name": "Ministry of Health and Welfare, Republic of Korea"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
29
]
],
"date-time": "2022-08-29T17:11:23Z",
"timestamp": 1661793083248
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
8,
1
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2022,
8,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 7,
"start": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T00:00:00Z",
"timestamp": 1659916800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac406/45287239/ofac406.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/9/8/ofac406/45612082/ofac406.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/9/8/ofac406/45612082/ofac406.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
8
]
]
},
"published-other": {
"date-parts": [
[
2022,
8,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1152/physiolgenomics.00089.2020",
"article-title": "The COVID-19 pandemic: a global health crisis",
"author": "Pollard",
"doi-asserted-by": "crossref",
"first-page": "549",
"journal-title": "Physiol Genomics",
"key": "2022082916501028800_ofac406-B1",
"volume": "52",
"year": "2020"
},
{
"article-title": "Coronavirus disease (COVID-19) dashboard",
"author": "World Health Organization",
"key": "2022082916501028800_ofac406-B2"
},
{
"article-title": "Living guidance for clinical management of COVID-19",
"author": "World Health Organization",
"key": "2022082916501028800_ofac406-B3"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "2022082916501028800_ofac406-B4",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "2022082916501028800_ofac406-B5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2022082916501028800_ofac406-B6",
"volume": "325",
"year": "2021"
},
{
"article-title": "Hospitalisation risk for Omicron cases in England",
"author": "Ferguson",
"key": "2022082916501028800_ofac406-B7"
},
{
"DOI": "10.1016/S1473-3099(22)00141-4",
"article-title": "Severity of Omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design",
"author": "Sheikh",
"doi-asserted-by": "crossref",
"first-page": "959",
"journal-title": "Lancet Infect Dis",
"key": "2022082916501028800_ofac406-B8",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)00017-4",
"article-title": "Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study",
"author": "Wolter",
"doi-asserted-by": "crossref",
"first-page": "437",
"journal-title": "Lancet",
"key": "2022082916501028800_ofac406-B9",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2022.101455",
"article-title": "Severe hospital events following symptomatic infection with SARS-CoV-2 Omicron and Delta variants in France, December 2021–January 2022: a retrospective, population-based, matched cohort study",
"author": "Auvigne",
"doi-asserted-by": "crossref",
"first-page": "101455",
"journal-title": "EClinicalMedicine",
"key": "2022082916501028800_ofac406-B10",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.1111/tmi.13752",
"article-title": "Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa",
"author": "Davies",
"doi-asserted-by": "crossref",
"first-page": "564",
"journal-title": "Trop Med Int Health",
"key": "2022082916501028800_ofac406-B11",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "2022082916501028800_ofac406-B12",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1101/2022.01.19.476892",
"article-title": "Molecular basis of broad neutralization against SARS-CoV-2 variants including Omicron by a human antibody",
"author": "Ju",
"doi-asserted-by": "publisher",
"key": "2022082916501028800_ofac406-B13",
"volume-title": "bioRxiv"
},
{
"DOI": "10.1002/eji.202249841",
"article-title": "Augmented neutralization of SARS-CoV-2 Omicron variant by boost vaccination and monoclonal antibodies",
"author": "Schulz",
"doi-asserted-by": "crossref",
"first-page": "970",
"journal-title": "Eur J Immunol",
"key": "2022082916501028800_ofac406-B14",
"volume": "52",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"article-title": "A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "288",
"journal-title": "Nat Commun",
"key": "2022082916501028800_ofac406-B15",
"volume": "12",
"year": "2021"
},
{
"article-title": "Celltrion’s monoclonal antibody treatment for COVID-19, regdanvimab (CT-P59) becomes the first authorized COVID-19 treatment approved from the Korean Ministry of Food and Drug Safety (MFDS)",
"author": "Business Wire",
"key": "2022082916501028800_ofac406-B16"
},
{
"article-title": "Regkirona (regdanvimab)",
"author": "European Medicines Agency",
"key": "2022082916501028800_ofac406-B17"
},
{
"DOI": "10.1016/j.clinthera.2021.08.009",
"article-title": "Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1706",
"journal-title": "Clin Ther",
"key": "2022082916501028800_ofac406-B18",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac053",
"article-title": "Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019",
"author": "Streinu-Cercel",
"doi-asserted-by": "crossref",
"first-page": "ofac053",
"journal-title": "Open Forum Infect Dis",
"key": "2022082916501028800_ofac406-B19",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1101/2022.07.14.500041",
"article-title": "Neutralization sensitivity of Omicron BA.2.75 to therapeutic monoclonal antibodies",
"author": "Yamasoba",
"doi-asserted-by": "publisher",
"key": "2022082916501028800_ofac406-B20",
"volume-title": "bioRxiv"
},
{
"DOI": "10.1038/s41467-021-24339-7",
"article-title": "CVnCov and CV2CoV protect human ACE2 transgenic mice from ancestral B BavPat1 and emerging B.1.351 SARS-CoV-2",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "4048",
"journal-title": "Nat Commun",
"key": "2022082916501028800_ofac406-B21",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s12979-020-00212-x",
"article-title": "Obesity and COVID-19: what makes obese host so vulnerable?",
"author": "Mohammad",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Immun Ageing",
"key": "2022082916501028800_ofac406-B22",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1002/dmrr.3377",
"article-title": "Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19)",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "e3377",
"journal-title": "Diabetes Metab Res Rev",
"key": "2022082916501028800_ofac406-B23",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.pcd.2020.08.015",
"article-title": "Diabetes and COVID-19: a pooled analysis related to disease severity and mortality",
"author": "Varikasuvu",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Prim Care Diabetes",
"key": "2022082916501028800_ofac406-B24",
"volume": "15",
"year": "2021"
},
{
"article-title": "American College of Cardiology. Mitigating ASCVD risk among those previously infected with COVID-19",
"author": "Naz",
"key": "2022082916501028800_ofac406-B25"
},
{
"article-title": "COVID-19 risk factors and risk groups",
"author": "European Centre for Disease Prevention and Control",
"key": "2022082916501028800_ofac406-B26"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2022082916501028800_ofac406-B27",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "2022082916501028800_ofac406-B28",
"volume": "385",
"year": "2021"
},
{
"article-title": "Tracking of variants: VOC Delta, relative variant genome frequency per region",
"author": "GISAID",
"key": "2022082916501028800_ofac406-B29"
},
{
"DOI": "10.1136/bmjopen-2020-040380",
"article-title": "Temporal profile and determinants of viral shedding and of viral clearance confirmation on nasopharyngeal swabs from SARS-CoV-2-positive subjects: a population-based prospective cohort study in Reggio Emilia, Italy",
"author": "Mancuso",
"doi-asserted-by": "crossref",
"first-page": "e040380",
"journal-title": "BMJ Open",
"key": "2022082916501028800_ofac406-B30",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.772320",
"article-title": "Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "772320",
"journal-title": "Front Immunol",
"key": "2022082916501028800_ofac406-B31",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2021.06.016",
"article-title": "Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Biochem Biophys Res Commun",
"key": "2022082916501028800_ofac406-B32",
"volume": "566",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2021.09.023",
"article-title": "The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Biochem Biophys Res Commun",
"key": "2022082916501028800_ofac406-B33",
"volume": "578",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-040638",
"article-title": "Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: a hospital case note review",
"author": "Perkin",
"doi-asserted-by": "crossref",
"first-page": "e040638",
"journal-title": "BMJ Open",
"key": "2022082916501028800_ofac406-B34",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s40615-020-00934-0",
"article-title": "Age, comorbid conditions, and racial disparities in COVID-19 outcomes",
"author": "Wiley",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "J Racial Ethn Health Disparities",
"key": "2022082916501028800_ofac406-B35",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/j.annepidem.2020.07.007",
"article-title": "Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics",
"author": "Rodriguez-Diaz",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Ann Epidemiol",
"key": "2022082916501028800_ofac406-B36",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"article-title": "Neutralizing monoclonal antibodies for treatment of COVID-19",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "382",
"journal-title": "Nat Rev Immunol",
"key": "2022082916501028800_ofac406-B37",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1056/NEJMp1802256",
"article-title": "Monoclonal antibodies for emerging infectious diseases—borrowing from history",
"author": "Marston",
"doi-asserted-by": "crossref",
"first-page": "1469",
"journal-title": "N Engl J Med",
"key": "2022082916501028800_ofac406-B38",
"volume": "378",
"year": "2018"
},
{
"article-title": "Coronavirus-19 (COVID-19): Covid-19 vaccination and domestic outbreaks (2.4.) [in Korean]",
"author": "Ministry of Health and Welfare",
"key": "2022082916501028800_ofac406-B39"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofac406/6657716"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "A Randomized Clinical Trial of Regdanvimab in High-Risk Patients With Mild-to-Moderate Coronavirus Disease 2019",
"type": "journal-article",
"volume": "9"
}