Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial
et al., eBioMedicine, doi:10.1016/j.ebiom.2022.104322, NCT04532931, Nov 2022
High COI low-risk patient RCT in South Africa, showing no significant differences with favipiravir plus nitazoxanide. There were no deaths and no COVID-19 hospitalizations for favipiravir plus nitazoxanide. More patients were seropositive at baseline in the treatment arm (28% vs 22%). Favipiravir 1600mg 12-hourly for 1 day, then 600mg 12-hourly for 6 days. Nitazoxanide 1000mg 12-hourly for 7 days.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Study covers artemisinin, nitazoxanide, and favipiravir.
|
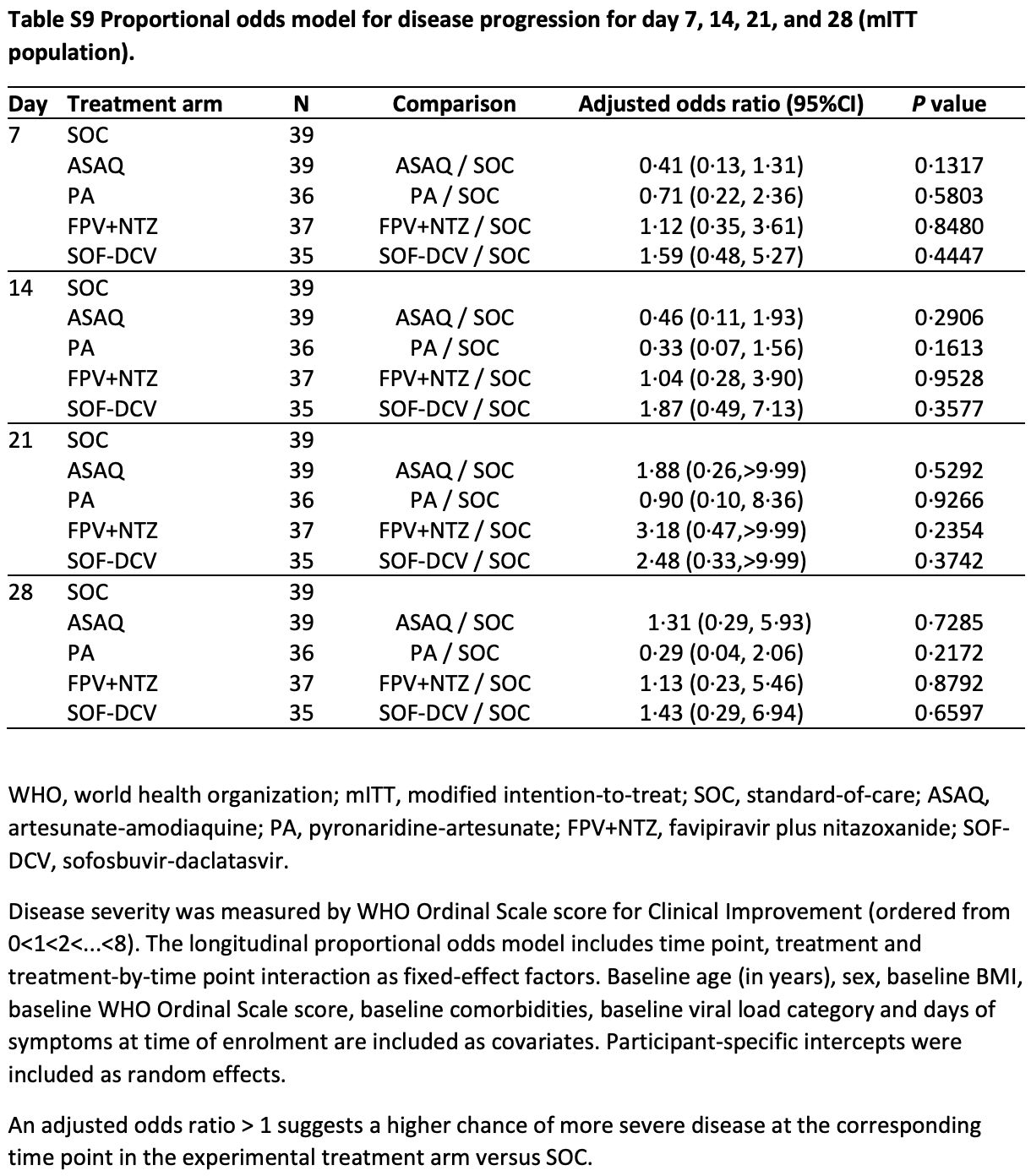
risk of progression, 13.0% higher, OR 1.13, p = 0.89, treatment 37, control 39, adjusted per study, day 28, Table S9, RR approximated with OR.
|
|
time to WHO zero score, 23.5% higher, HR 1.23, p = 0.42, treatment 37, control 39, inverted to make HR<1 favor treatment, Cox proportional hazards, Table S10.
|
|
risk of no viral clearance, 66.7% higher, RR 1.67, p = 0.13, treatment 27 of 37 (73.0%), control 25 of 38 (65.8%), adjusted per study, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Chandiwana et al., 1 Nov 2022, Randomized Controlled Trial, South Africa, peer-reviewed, mean age 34.9, 16 authors, study period 3 September, 2020 - 23 August, 2021, average treatment delay 2.6 days, this trial uses multiple treatments in the treatment arm (combined with nitazoxanide) - results of individual treatments may vary, trial NCT04532931 (history).
Contact: nchandiwana@ezintsha.org.
Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial
eBioMedicine, doi:10.1016/j.ebiom.2022.104322
Background This exploratory study investigated four repurposed anti-infective drug regimens in outpatients with COVID-19. Methods This phase 2, single centre, randomised, open-label, clinical trial was conducted in South Africa between 3rd September 2020 and 23rd August 2021. Symptomatic outpatients aged 18-65 years, with RT-PCR confirmed SARS-CoV-2 infection were computer randomised (1:1:1:1:1) to standard-of-care (SOC) with paracetamol, or SOC plus artesunate-amodiaquine (ASAQ), pyronaridine-artesunate (PA), favipiravir plus nitazoxanide (FPV + NTZ), or sofosbuvir-daclatasvir (SOF-DCV). The primary endpoint was the incidence of viral clearance, i.e., the proportion of patients with a negative SARS-CoV-2 RT-PCR on day 7, compared to SOC using a log-binomial model in the modified intention-to-treat (mITT) population. Findings The mITT population included 186 patients: mean age (SD) 34.9 (10.3) years, body weight 78.2 (17.1) kg. Day 7 SARS-CoV-2 clearance rates (n/N; risk ratio [95% CI]) were: SOC 34.2% (13/38), ASAQ 38.5% (15/39; 0.80 [0.44, 1.47]), PA 30.3% (10/33; 0.69 [0.37, 1.29]), FPV + NTZ 27.0% (10/37; 0.60 [0.31, 1.18]) and SOF-DCV 23.5% (8/34; 0.47 [0.22, 1.00]). Three lower respiratory tract infections occurred (PA 6.1% [2/33]; SOF-DCV 2.9% [1/34]); two required hospitalisation (PA, SOF-DCV). There were no deaths. Adverse events occurred in 55.3% (105/190) of patients, including one serious adverse event (pancytopenia; FPV + NTZ). Interpretation There was no statistical difference in viral clearance for any regimen compared to SOC. All treatments were well tolerated.
Appendix A. Supplementary data Supplementary data related to this article can be found at https://doi. org/10.1016/j.ebiom.2022.104322.
References
Amanat, White, Miorin, An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening, Curr Protoc Microbiol
Arshad, Pertinez, Box, Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther
Augustin, Schommers, Stecher, Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study, Lancet Reg Health Eur
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med
Bosaeed, Alharbi, Mahmoud, Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicenter, placebo-controlled trial clinical trial, Clin Microbiol Infect
Bullard-Feibelman, Govero, Zhu, The FDA-approved drug sofosbuvir inhibits Zika virus infection, Antiviral Res
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Carmo, Pereira-Vaz, Mota, Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19, J Med Virol
Carrat, Duval, Tubach, Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households, Antivir Ther
Chen, Zhang, Huang, Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial, Front Pharmacol
De Freitas, Higa, Sacramento, Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo, PLoS Negl Trop Dis
Delang, Abdelnabi, Neyts, Favipiravir as a potential countermeasure against neglected and emerging RNA viruses, Antiviral Res
Duparc, Borghini-Fuhrer, Craft, Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials, Malar J
El Kassas, Abdeen, Omran, Safety and efficacy of sofosbuvir/ledipasvir and sofosbuvir/daclatasvir in the treatment of hepatitis C in patients with decompensated cirrhosis, Eur J Gastroenterol Hepatol
Ferreira, Reis, De Freitas, Beyond members of the flaviviridae family, sofosbuvir also inhibits Chikungunya virus replication, Antimicrobial Agents Chemother
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Gan, Lim, Chee, Yusof, Heh, Sofosbuvir as treatment against dengue?, Chem Biol Drug Des
Ghaffar, Naghi, Gawad, Safety and efficacy of combined sofosbuvir/daclatasvir treatment of children and adolescents with chronic hepatitis C Genotype 4, J Viral Hepat
Gulhan, Eryuksel, Idriz Oglu, Pharmacokinetic characterization of favipiravir in patients with COVID-19, Br J Clin Pharmacol
Kasgari, Moradi, Shabani, Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial, J Antimicrob Chemother
Kim, Read, Fauci, Therapy for early COVID-19: a critical need, JAMA
Krishna, Wang, Repurposing antimalarials to tackle the COVID-19 pandemic, Trends Parasitol
Li, Taljaard, Van Den Heuvel, An introduction to multiplicity issues in clinical trials: the what, why, when and how, Int J Epidemiol
Lou, Liu, Yao, Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in covid-19 patients: an exploratory randomized, controlled trial, Eur J Pharm Sci
Mahase, Covid-19: UK becomes first country to authorise antiviral molnupiravir, BMJ
Mandorfer, Schwabl, Steiner, Interferon-free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus-coinfected patients with advanced liver disease, AIDS
Mobarak, Salasi, Hormati, Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER), J Antimicrob Chemother
Munoz-Fontela, Dowling, Funnell, Animal models for COVID-19, Nature
Parums, Editorial: current status of oral antiviral drug treatments for SARS-CoV-2 infection in non-hospitalized patients, Med Sci Monit
Pellicelli, Messina, Giannelli, High efficacy and safety of flat-dose ribavirin plus sofosbuvir/daclatasvir in genotype 3 cirrhotic patients, Gut Liver
Pepperrell, Pilkington, Owen, Wang, Hill, Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19, J Virus Erad
Powers, Guerrero, Leidy, Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza, BMC Infect Dis
Puhl, Gomes, Damasceno, Pyronaridine protects against SARS-CoV-2 infection in mouse, ACS Infect Dis
Rajoli, Pertinez, Arshad, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol
Roozbeh, Saeedi, Alizadeh-Navaei, Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial, J Antimicrob Chemother
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Ruzhentsova, Oseshnyuk, Soluyanova, Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19, Am J Transl Res
Srinivasan, Rao, Understanding the clinical utility of favipiravir (T-705) in coronavirus disease of 2019: a review, Ther Adv Infect Dis
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, openlabel, multicenter, phase 3 clinical trial, Int J Infect Dis
Walker, Fitzgerald, Saunders, An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2, Clin Pharmacol Ther
Zwang, Dorsey, Djimde, Clinical tolerability of artesunate-amodiaquine versus comparator treatments for uncomplicated falciparum malaria: an individual-patient analysis of eight randomized controlled trials in sub-Saharan Africa, Malar J
DOI record:
{
"DOI": "10.1016/j.ebiom.2022.104322",
"ISSN": [
"2352-3964"
],
"URL": "http://dx.doi.org/10.1016/j.ebiom.2022.104322",
"alternative-id": [
"S2352396422005047"
],
"article-number": "104322",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eBioMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ebiom.2022.104322"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7866-2651",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chandiwana",
"given": "Nomathemba",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kruger",
"given": "Chelsea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnstone",
"given": "Hilary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chughlay",
"given": "Mohamed Farouk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ju",
"given": "Chung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Byungsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dineka",
"given": "Yengiwe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arbe-Barnes",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hill",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Windgassen",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abla",
"given": "Nada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marrast",
"given": "Anne Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duparc",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francois Venter",
"given": "Willem Daniel",
"sequence": "additional"
}
],
"container-title": "eBioMedicine",
"container-title-short": "eBioMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T17:38:01Z",
"timestamp": 1667324281000
},
"deposited": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T17:38:33Z",
"timestamp": 1667324313000
},
"indexed": {
"date-parts": [
[
2022,
11,
2
]
],
"date-time": "2022-11-02T04:50:30Z",
"timestamp": 1667364630222
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T00:00:00Z",
"timestamp": 1669852800000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
12
]
],
"date-time": "2022-10-12T00:00:00Z",
"timestamp": 1665532800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2352396422005047?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2352396422005047?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "104322",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.ebiom.2022.104322_bib1",
"series-title": "Coronavirus resource center",
"year": "2021"
},
{
"article-title": "Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study",
"author": "Augustin",
"journal-title": "Lancet Reg Health Eur",
"key": "10.1016/j.ebiom.2022.104322_bib2",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3851/IMP2128",
"article-title": "Effect of oseltamivir, zanamivir or oseltamivir-zanamivir combination treatments on transmission of influenza in households",
"author": "Carrat",
"doi-asserted-by": "crossref",
"first-page": "1085",
"journal-title": "Antivir Ther",
"key": "10.1016/j.ebiom.2022.104322_bib3",
"volume": "17",
"year": "2012"
},
{
"DOI": "10.1001/jama.2020.22813",
"article-title": "Therapy for early COVID-19: a critical need",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "2149",
"journal-title": "JAMA",
"key": "10.1016/j.ebiom.2022.104322_bib4",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.12659/MSM.935952",
"article-title": "Editorial: current status of oral antiviral drug treatments for SARS-CoV-2 infection in non-hospitalized patients",
"author": "Parums",
"doi-asserted-by": "crossref",
"journal-title": "Med Sci Monit",
"key": "10.1016/j.ebiom.2022.104322_bib5",
"volume": "28",
"year": "2022"
},
{
"key": "10.1016/j.ebiom.2022.104322_bib6",
"series-title": "Therapeutic management of nonhospitalized adults with COVID-19",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2697",
"article-title": "Covid-19: UK becomes first country to authorise antiviral molnupiravir",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "2697",
"journal-title": "BMJ",
"key": "10.1016/j.ebiom.2022.104322_bib7",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritization of anti-SARS-Cov-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics",
"author": "Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ebiom.2022.104322_bib8",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1016/j.pt.2020.10.003",
"article-title": "Repurposing antimalarials to tackle the COVID-19 pandemic",
"author": "Krishna",
"doi-asserted-by": "crossref",
"first-page": "8",
"journal-title": "Trends Parasitol",
"key": "10.1016/j.ebiom.2022.104322_bib9",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2787-6",
"article-title": "Animal models for COVID-19",
"author": "Munoz-Fontela",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2022.104322_bib10",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.1186/1475-2875-12-70",
"article-title": "Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials",
"author": "Duparc",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "Malar J",
"key": "10.1016/j.ebiom.2022.104322_bib11",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1186/1475-2875-11-260",
"article-title": "Clinical tolerability of artesunate-amodiaquine versus comparator treatments for uncomplicated falciparum malaria: an individual-patient analysis of eight randomized controlled trials in sub-Saharan Africa",
"author": "Zwang",
"doi-asserted-by": "crossref",
"first-page": "260",
"journal-title": "Malar J",
"key": "10.1016/j.ebiom.2022.104322_bib12",
"volume": "11",
"year": "2012"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/j.ebiom.2022.104322_bib13",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"article-title": "Nitazoxanide: a first-in-class broad-spectrum antiviral agent",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ebiom.2022.104322_bib14",
"volume": "110",
"year": "2014"
},
{
"DOI": "10.1097/QAD.0000000000001020",
"article-title": "Interferon-free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus-coinfected patients with advanced liver disease",
"author": "Mandorfer",
"doi-asserted-by": "crossref",
"first-page": "1039",
"journal-title": "AIDS",
"key": "10.1016/j.ebiom.2022.104322_bib15",
"volume": "30",
"year": "2016"
},
{
"DOI": "10.1371/journal.pntd.0007072",
"article-title": "Yellow fever virus is susceptible to sofosbuvir both in vitro and in vivo",
"author": "de Freitas",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Negl Trop Dis",
"key": "10.1016/j.ebiom.2022.104322_bib16",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1016/j.antiviral.2016.11.023",
"article-title": "The FDA-approved drug sofosbuvir inhibits Zika virus infection",
"author": "Bullard-Feibelman",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ebiom.2022.104322_bib17",
"volume": "137",
"year": "2017"
},
{
"DOI": "10.1111/cbdd.13091",
"article-title": "Sofosbuvir as treatment against dengue?",
"author": "Gan",
"doi-asserted-by": "crossref",
"first-page": "448",
"journal-title": "Chem Biol Drug Des",
"key": "10.1016/j.ebiom.2022.104322_bib18",
"volume": "91",
"year": "2018"
},
{
"DOI": "10.1128/AAC.01389-18",
"article-title": "Beyond members of the flaviviridae family, sofosbuvir also inhibits Chikungunya virus replication",
"author": "Ferreira",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrobial Agents Chemother",
"key": "10.1016/j.ebiom.2022.104322_bib19",
"volume": "63",
"year": "2019"
},
{
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"first-page": "1192",
"journal-title": "Engineering (Beijing)",
"key": "10.1016/j.ebiom.2022.104322_bib20",
"volume": "6",
"year": "2020"
},
{
"article-title": "Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial",
"author": "Chen",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ebiom.2022.104322_bib21",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/cpmc.108",
"article-title": "An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening",
"author": "Amanat",
"doi-asserted-by": "crossref",
"first-page": "e108",
"journal-title": "Curr Protoc Microbiol",
"key": "10.1016/j.ebiom.2022.104322_bib23",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1186/s12879-015-1330-0",
"article-title": "Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza",
"author": "Powers",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.ebiom.2022.104322_bib24",
"volume": "16",
"year": "2016"
},
{
"key": "10.1016/j.ebiom.2022.104322_bib25",
"series-title": "WHO R&D Blueprint novel coronavirus COVID-19 therapeutic trial synopsis",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ebiom.2022.104322_bib26",
"volume": "20",
"year": "2020"
},
{
"article-title": "An introduction to multiplicity issues in clinical trials: the what, why, when and how",
"author": "Li",
"first-page": "746",
"journal-title": "Int J Epidemiol",
"key": "10.1016/j.ebiom.2022.104322_bib27",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.1016/j.antiviral.2018.03.003",
"article-title": "Favipiravir as a potential countermeasure against neglected and emerging RNA viruses",
"author": "Delang",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ebiom.2022.104322_bib28",
"volume": "153",
"year": "2018"
},
{
"DOI": "10.1016/S2055-6640(20)30017-0",
"article-title": "Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19",
"author": "Pepperrell",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "J Virus Erad",
"key": "10.1016/j.ebiom.2022.104322_bib29",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1097/MEG.0000000000002287",
"article-title": "Safety and efficacy of sofosbuvir/ledipasvir and sofosbuvir/daclatasvir in the treatment of hepatitis C in patients with decompensated cirrhosis",
"author": "El Kassas",
"doi-asserted-by": "crossref",
"first-page": "e877",
"journal-title": "Eur J Gastroenterol Hepatol",
"key": "10.1016/j.ebiom.2022.104322_bib30",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.5009/gnl18269",
"article-title": "High efficacy and safety of flat-dose ribavirin plus sofosbuvir/daclatasvir in genotype 3 cirrhotic patients",
"author": "Pellicelli",
"doi-asserted-by": "crossref",
"first-page": "357",
"journal-title": "Gut Liver",
"key": "10.1016/j.ebiom.2022.104322_bib31",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1111/jvh.13032",
"article-title": "Safety and efficacy of combined sofosbuvir/daclatasvir treatment of children and adolescents with chronic hepatitis C Genotype 4",
"author": "Abdel Ghaffar",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "J Viral Hepat",
"key": "10.1016/j.ebiom.2022.104322_bib32",
"volume": "26",
"year": "2019"
},
{
"DOI": "10.1021/acsinfecdis.2c00091",
"article-title": "Pyronaridine protects against SARS-CoV-2 infection in mouse",
"author": "Puhl",
"doi-asserted-by": "crossref",
"first-page": "1147",
"journal-title": "ACS Infect Dis",
"key": "10.1016/j.ebiom.2022.104322_bib33",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"article-title": "Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicenter, placebo-controlled trial clinical trial",
"author": "Bosaeed",
"doi-asserted-by": "crossref",
"first-page": "602",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.ebiom.2022.104322_bib34",
"volume": "28",
"year": "2022"
},
{
"article-title": "Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19",
"author": "Ruzhentsova",
"first-page": "12575",
"journal-title": "Am J Transl Res",
"key": "10.1016/j.ebiom.2022.104322_bib35",
"volume": "13",
"year": "2021"
},
{
"article-title": "Understanding the clinical utility of favipiravir (T-705) in coronavirus disease of 2019: a review",
"author": "Srinivasan",
"journal-title": "Ther Adv Infect Dis",
"key": "10.1016/j.ebiom.2022.104322_bib36",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"article-title": "Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in covid-19 patients: an exploratory randomized, controlled trial",
"author": "Lou",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Pharm Sci",
"key": "10.1016/j.ebiom.2022.104322_bib37",
"volume": "157",
"year": "2021"
},
{
"DOI": "10.1111/bcp.15227",
"article-title": "Pharmacokinetic characterization of favipiravir in patients with COVID-19",
"author": "Gulhan",
"doi-asserted-by": "crossref",
"first-page": "3516",
"journal-title": "Br J Clin Pharmacol",
"key": "10.1016/j.ebiom.2022.104322_bib38",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1111/bcp.14619",
"article-title": "Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis",
"author": "Rajoli",
"doi-asserted-by": "crossref",
"first-page": "2078",
"journal-title": "Br J Clin Pharmacol",
"key": "10.1016/j.ebiom.2022.104322_bib39",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2463",
"article-title": "An open label, adaptive, phase 1 trial of high-dose oral nitazoxanide in healthy volunteers: an antiviral candidate for SARS-CoV-2",
"author": "Walker",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ebiom.2022.104322_bib40",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkaa501",
"article-title": "Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial",
"author": "Roozbeh",
"doi-asserted-by": "crossref",
"first-page": "753",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.ebiom.2022.104322_bib41",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkab433",
"article-title": "Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER)",
"author": "Mobarak",
"doi-asserted-by": "crossref",
"first-page": "758",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.ebiom.2022.104322_bib42",
"volume": "77",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkaa332",
"article-title": "Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial",
"author": "Abbaspour Kasgari",
"doi-asserted-by": "crossref",
"first-page": "3373",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.ebiom.2022.104322_bib43",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26103",
"article-title": "Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19",
"author": "Carmo",
"doi-asserted-by": "crossref",
"first-page": "2227",
"journal-title": "J Med Virol",
"key": "10.1016/j.ebiom.2022.104322_bib44",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ebiom.2022.104322_bib45",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ebiom.2022.104322_bib46",
"volume": "386",
"year": "2021"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2352396422005047"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Safety and efficacy of four drug regimens versus standard-of-care for the treatment of symptomatic outpatients with COVID-19: A randomised, open-label, multi-arm, phase 2 clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "86"
}