High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00545-2, CONV-ERT, NCT04621123, Feb 2022
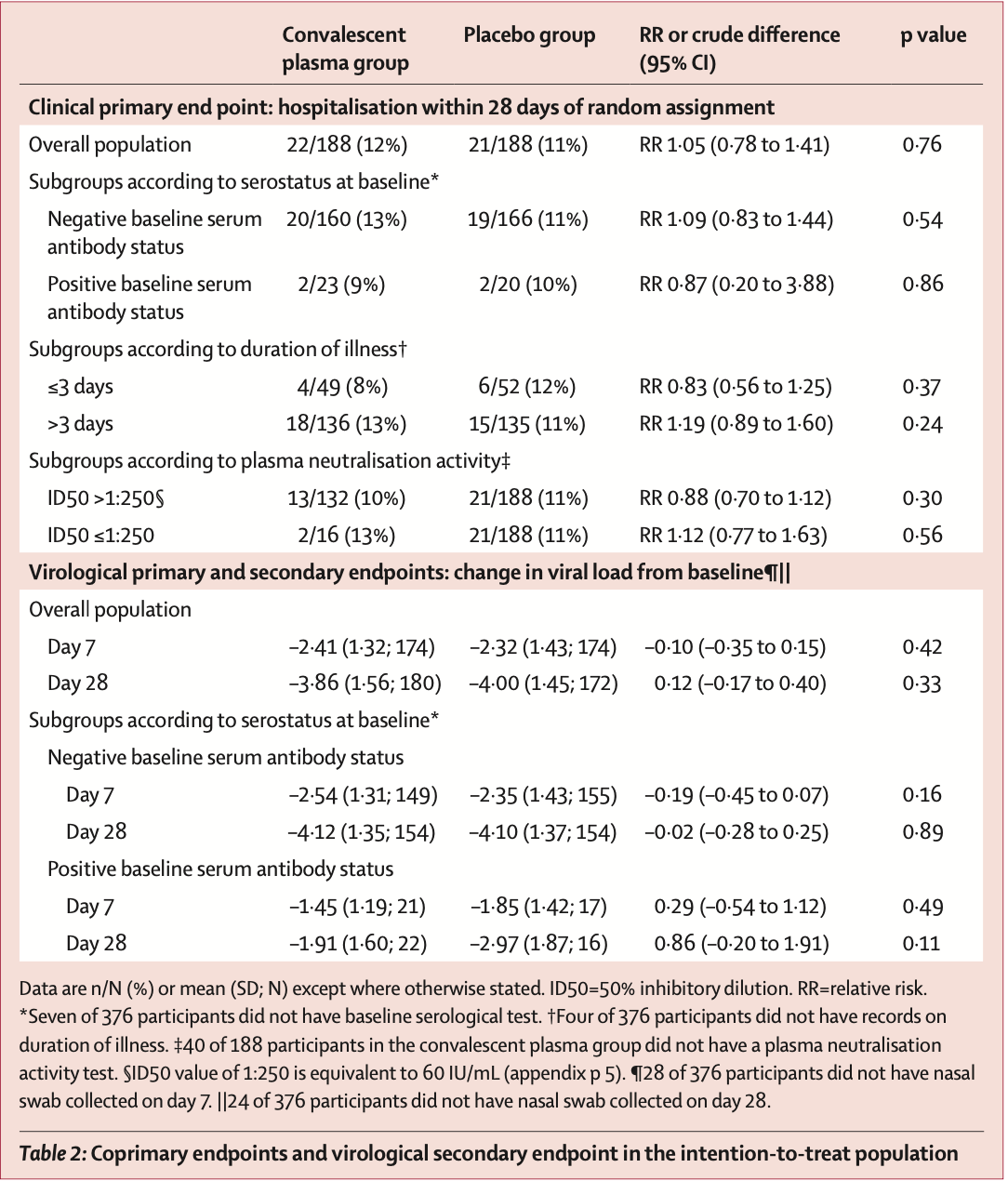
RCT 188 convalescent plasma and 188 control patients, showing no significant difference in outcomes.
|
risk of death, 80.0% lower, RR 0.20, p = 0.50, treatment 0 of 188 (0.0%), control 2 of 188 (1.1%), NNT 94, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 4.8% higher, RR 1.05, p = 1.00, treatment 22 of 188 (11.7%), control 21 of 188 (11.2%).
|
|
risk of no recovery, 5.0% higher, HR 1.05, p = 0.67, treatment 188, control 188, time to symptom resolution.
|
|
viral load, 3.6% higher, relative load 1.04, p = 0.33, treatment 188, control 188, relative change in viral load, day 28.
|
|
viral load, 3.7% lower, relative load 0.96, p = 0.42, treatment 188, control 188, relative change in viral load, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Alemany et al., 9 Feb 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, median age 56.0, 108 authors, study period 10 November, 2020 - 28 July, 2021, trial NCT04621123 (history) (CONV-ERT).
Contact: aalemany@flsida.org.
High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00545-2
Background Convalescent plasma has been proposed as an early treatment to interrupt the progression of early COVID-19 to severe disease, but there is little definitive evidence. We aimed to assess whether early treatment with convalescent plasma reduces the risk of hospitalisation and reduces SARS-CoV-2 viral load among outpatients with COVID-19.
Methods We did a multicentre, double-blind, randomised, placebo-controlled trial in four health-care centres in Catalonia, Spain. Adult outpatients aged 50 years or older with the onset of mild COVID-19 symptoms 7 days or less before randomisation were eligible for enrolment. Participants were randomly assigned (1:1) to receive one intravenous infusion of either 250-300 mL of ABO-compatible high anti-SARS-CoV-2 IgG titres (EUROIMMUN ratio ≥6) methylene blue-treated convalescent plasma (experimental group) or 250 mL of sterile 0•9% saline solution (control). Randomisation was done with the use of a central web-based system with concealment of the trial group assignment and no stratification. To preserve masking, we used opaque tubular bags that covered the investigational product and the infusion catheter. The coprimary endpoints were the incidence of hospitalisation within 28 days from baseline and the mean change in viral load (in log 10 copies per mL) in nasopharyngeal swabs from baseline to day 7. The trial was stopped early following a data safety monitoring board recommendation because more than 85% of the target population had received a COVID-19 vaccine. Primary efficacy analyses were done in the intentionto-treat population, safety was assessed in all patients who received the investigational product. This study is registered with ClinicalTrials.gov, NCT04621123. Findings Between Nov 10, 2020, and July 28, 2021, we assessed 909 patients with confirmed COVID-19 for inclusion in the trial, 376 of whom were eligible and were randomly assigned to treatment (convalescent plasma n=188 [serum antibody-negative n=160]; placebo n=188 [serum antibody-negative n=166]). Median age was 56 years (IQR 52-62) and the mean symptom duration was 4•4 days (SD 1•4) before random assignment. In the intention-to-treat population, hospitalisation within 28 days from baseline occurred in 22 (12%) participants who received convalescent plasma versus 21 (11%) who received placebo (relative risk 1•05 [95% CI 0•78 to 1•41]). The mean change in viral load from baseline to day 7 was -2•41 log 10 copies per mL (SD 1•32) with convalescent plasma and -2•32 log 10 copies per mL (1•43) with placebo (crude difference -0•10 log 10 copies per mL [95% CI -0•35 to 0•15]). One participant with mild COVID-19 developed a thromboembolic event 7 days after convalescent plasma infusion, which was reported as a serious adverse event possibly related to COVID-19 or to the experimental intervention. Interpretation Methylene blue-treated convalescent plasma did not prevent progression from mild to severe illness and did not reduce viral load in..
company, Grifols Worldwide Operations (Dublin, Ireland), and the Crowdfunding campaign, YoMeCorono. The study received support from the Hospital Universitari Germans Trias i Pujol, and Banc de Sang i Teixits de Catalunya. We thank Gerard Carot-Sans for providing medical writing support with manuscript preparation and Roser Escrig for her support in the study design and medical writing assistance with the study documentation. We also thank Laia Bertran, Mireia Clua, Jordi Mitjà, Claudia Laporte, Sergi Gavilan, Joan Mercado, and Enric Nieto for the operational and financial management of the project. We thank the personnel from the Fight Aids and Infectious Diseases Foundation for their support in administration, human resources, and supply chain management. We thank the independent data safety monitoring board for their time and dedication: Cinta Hierro (Catalan Institute of Oncology, Badalona, Spain), Natalia Tovar (Hospital Clinic, Barcelona, Spain), Binh Ngo (University of Southern California, Los Angeles, CA, USA), David Boulware (University of Minnesota, Minneapolis, MN, USA), and Robin Mogg (Bill and Melinda Gates Research Institute, Seattle, WA, USA). We thank the reviewers for substantial comments that helped improve and clarify the strengths and drawbacks of the experimental intervention and study methodology.
References
Abani, Abbas, Abbas, Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Alqahtani, Abdulrahman, Almadani, Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Sci Rep
Avendaño-Solá, Ramos-Martínez, Muñez-Rubio, A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest
Bajpai, Kumar, Maheshwari, Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial, medRxiv, doi:10.1101/2020.10.25.20219337
Balcells, Rojas, Corre, Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial, PLoS Med
Bratcher-Bowman, Convalescent plasma EUA letter of authorization March 9
Bégin, Callum, Jamula, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, bioRxiv, doi:10.1101/2021.12.07.470392
Deeks, Higgins, Statistical algorithms in Review Manager 5
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med
Focosi, Franchini, Pirofski, COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors, Viruses
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Horby, Mafham, Peto, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, doi:10.1101/2021.06.15.21258542
Janiaud, Axfors, Schmitt, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis, JAMA
Katz, A little) clarity on convalescent plasma for COVID-19, N Engl J Med
Kermali, Khalsa, Pillai, Ismail, Harky, The role of biomarkers in diagnosis of COVID-19-a systematic review, Life Sci
Korley, Durkalski-Mauldin, Yeatts, Early convalescent plasma for high-risk outpatients with COVID-19, N Engl J Med
Kostin, Lundgren, Bulanov, Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma, Vox Sang
Kunze, Johnson, Van Helmond, Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors, Nat Commun
Larrea, Castro, Navarro, Preservation of anti-SARS-CoV-2 neutralising antibodies in convalescent plasma after pathogen reduction with methylene blue and visible light, Blood Transfus, doi:10.2450/2021.0136-21
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and lifethreatening COVID-19: a randomized clinical trial, JAMA
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med
Mair-Jenkins, Saavedra-Campos, Baillie, The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis, J Infect Dis
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
O'donnell, Grinsztejn, Cummings, A randomized, double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J Clin Invest
Raster, Zimmermann, Wesche, Aurich, Greinacher et al., Effect of methylene blue pathogen inactivation on the integrity of immunoglobulin M and G, Transfus Med Hemother
Ray, Paul, Bandopadhyay, Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial, medRxiv, doi:10.1101/2020.11.25.20237883
Ross, Photodynamic action of methylene blue on antipneumococcal serum, J Immunol
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med
Trinité, Pradenas, Marfil, Previous SARS-CoV-2 infection increases B.1.1.7 cross-neutralization by vaccinated individuals, Viruses
Trinité, Tarrés-Freixas, Rodon, SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity, Sci Rep
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Wilhelm, Widera, Grikscheit, Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies, medRxiv, doi:10.1101/2021.12.07.21267432
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00545-2",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00545-2",
"alternative-id": [
"S2213260021005452"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00545-2"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(22)00050-9"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Alemany",
"given": "Andrea",
"sequence": "first"
},
{
"affiliation": [],
"family": "Millat-Martinez",
"given": "Pere",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Corbacho-Monné",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malchair",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ouchi",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruiz-Comellas",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Morros",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez Codina",
"given": "Joana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amado Simon",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Videla",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costes",
"given": "Gèlia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Capdevila-Jáuregui",
"given": "Mar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torrano-Soler",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "San José",
"given": "Alba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonet Papell",
"given": "Glòria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puig",
"given": "Jordi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otero",
"given": "Aurema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruibal Suarez",
"given": "Jose Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zarauza Pellejero",
"given": "Alvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Llopis Roca",
"given": "Ferran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodriguez Cortez",
"given": "Orlando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia Garcia",
"given": "Vanesa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vidal-Alaball",
"given": "Josep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Millan",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Contreras",
"given": "Enric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grifols",
"given": "Joan-Ramon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ancochea",
"given": "Àgueda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galvan-Femenia",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piccolo Ferreira",
"given": "Francini",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonet",
"given": "Mireia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cantoni",
"given": "Jordi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prat",
"given": "Núria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ara",
"given": "Jordi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forcada Arcarons",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farré",
"given": "Magí",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pradenas",
"given": "Edwards",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco",
"given": "Julià",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Àngel Rodriguez-Arias",
"given": "Miquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández Rivas",
"given": "Gema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marks",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bassat",
"given": "Quique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baro",
"given": "Bàrbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clotet",
"given": "Bonaventura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mitjà",
"given": "Oriol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Susana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallardo",
"given": "Mireia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ubals",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González-Beiras",
"given": "Camila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vall-Mayans",
"given": "Martí",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suñer",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laporte-Villar",
"given": "Clàudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nieto",
"given": "Aroa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Comas-Leon",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiménez",
"given": "Zahida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Viaplana",
"given": "Ferran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delgado-Capel",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díez Sánchez",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pons Barber",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzalez Ruiz",
"given": "Cristian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navarrete Gonzalez",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González García",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vivero Larraza",
"given": "Ainhoa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carceles Peiró",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roquer López",
"given": "Clàudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robert",
"given": "Neus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palet",
"given": "Carles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gudiol",
"given": "Carlota",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casares Gonzalez",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arcos Vila",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores Aguilera",
"given": "Begoña",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Sevilla",
"given": "Graciela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dastis Arias",
"given": "Macarena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roca Font",
"given": "Judit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrasco Matos",
"given": "Katherine M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saüch Valmaña",
"given": "Glòria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vidal Obradors",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarres García",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curriu Sabatès",
"given": "Margarida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nieto Rodríguez",
"given": "Raquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Línio",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fornos",
"given": "Míriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casamitjana",
"given": "Natàlia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alonso",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez",
"given": "Núria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maglio",
"given": "Laura Analía",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Comellas Fernandez",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González",
"given": "Maria Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bravo",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sauleda Oliveras",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vertiz",
"given": "Tatiana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benavent",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bianco",
"given": "Andrea Sofia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verdaguer",
"given": "Joaquim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Briones Zambrano",
"given": "Ney Nicanor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viozquez Meya",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández",
"given": "Águeda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casaña Lopez",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bordoy",
"given": "Antoni E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González Soler",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giménez",
"given": "Montserrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "París",
"given": "Alexa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marfil",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trinité",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grau",
"given": "Eulàlia",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T23:38:25Z",
"timestamp": 1644449905000
},
"deposited": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T00:19:37Z",
"timestamp": 1646180377000
},
"indexed": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T05:50:54Z",
"timestamp": 1660974654959
},
"is-referenced-by-count": 11,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021005452?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021005452?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "278-288",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMe2035678",
"article-title": "(A little) clarity on convalescent plasma for COVID-19",
"author": "Katz",
"doi-asserted-by": "crossref",
"first-page": "666",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib1",
"volume": "384",
"year": "2021"
},
{
"article-title": "Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(21)00545-2_bib2",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib3",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00545-2_bib4",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib6",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"article-title": "Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "130",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(21)00545-2_bib7",
"volume": "593",
"year": "2021"
},
{
"article-title": "Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies",
"author": "Wilhelm",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(21)00545-2_bib8",
"year": "2021"
},
{
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"journal-title": "bioRxiv",
"key": "10.1016/S2213-2600(21)00545-2_bib9",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiu396",
"article-title": "The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis",
"author": "Mair-Jenkins",
"doi-asserted-by": "crossref",
"first-page": "80",
"journal-title": "J Infect Dis",
"key": "10.1016/S2213-2600(21)00545-2_bib10",
"volume": "211",
"year": "2015"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial",
"author": "Abani",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00545-2_bib11",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis",
"author": "Janiaud",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00545-2_bib12",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00545-2_bib13",
"volume": "324",
"year": "2020"
},
{
"article-title": "Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)",
"author": "Agarwal",
"journal-title": "BMJ",
"key": "10.1016/S2213-2600(21)00545-2_bib14",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in COVID-19 severe pneumonia",
"author": "Simonovich",
"doi-asserted-by": "crossref",
"first-page": "619",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S2213-2600(21)00545-2_bib16",
"volume": "12",
"year": "2021"
},
{
"article-title": "Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial",
"author": "Bajpai",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(21)00545-2_bib17",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-89444-5",
"article-title": "Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease",
"author": "AlQahtani",
"doi-asserted-by": "crossref",
"journal-title": "Sci Rep",
"key": "10.1016/S2213-2600(21)00545-2_bib18",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1371/journal.pmed.1003415",
"article-title": "Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial",
"author": "Balcells",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Med",
"key": "10.1016/S2213-2600(21)00545-2_bib19",
"volume": "18",
"year": "2021"
},
{
"article-title": "Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial",
"author": "Ray",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(21)00545-2_bib20",
"year": "2020"
},
{
"DOI": "10.1172/JCI152740",
"article-title": "A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia",
"author": "Avendaño-Solá",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/S2213-2600(21)00545-2_bib21",
"volume": "131",
"year": "2021"
},
{
"article-title": "A randomized, double-blind controlled trial of convalescent plasma in adults with severe COVID-19",
"author": "O'Donnell",
"journal-title": "J Clin Invest",
"key": "10.1016/S2213-2600(21)00545-2_bib22",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early convalescent plasma for high-risk outpatients with COVID-19",
"author": "Korley",
"doi-asserted-by": "crossref",
"first-page": "1951",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib23",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-25113-5",
"article-title": "Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors",
"author": "Kunze",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "10.1016/S2213-2600(21)00545-2_bib24",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe COVID-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00545-2_bib25",
"volume": "384",
"year": "2021"
},
{
"author": "Bratcher-Bowman",
"key": "10.1016/S2213-2600(21)00545-2_bib27"
},
{
"DOI": "10.3390/v13061135",
"article-title": "Previous SARS-CoV-2 infection increases B.1.1.7 cross-neutralization by vaccinated individuals",
"author": "Trinité",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/S2213-2600(21)00545-2_bib28",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2020.117788",
"article-title": "The role of biomarkers in diagnosis of COVID-19—a systematic review",
"author": "Kermali",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci",
"key": "10.1016/S2213-2600(21)00545-2_bib29",
"volume": "254",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S2213-2600(21)00545-2_bib30",
"volume": "20",
"year": "2020"
},
{
"author": "Deeks",
"key": "10.1016/S2213-2600(21)00545-2_bib32"
},
{
"DOI": "10.1038/s41598-021-81862-9",
"article-title": "SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity",
"author": "Trinité",
"doi-asserted-by": "crossref",
"journal-title": "Sci Rep",
"key": "10.1016/S2213-2600(21)00545-2_bib33",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1111/vox.13056",
"article-title": "Impact of pathogen reduction methods on immunological properties of the COVID-19 convalescent plasma",
"author": "Kostin",
"doi-asserted-by": "crossref",
"first-page": "665",
"journal-title": "Vox Sang",
"key": "10.1016/S2213-2600(21)00545-2_bib34",
"volume": "116",
"year": "2021"
},
{
"article-title": "Preservation of anti-SARS-CoV-2 neutralising antibodies in convalescent plasma after pathogen reduction with methylene blue and visible light",
"author": "Larrea",
"journal-title": "Blood Transfus",
"key": "10.1016/S2213-2600(21)00545-2_bib35",
"year": "2021"
},
{
"DOI": "10.1159/000514485",
"article-title": "Effect of methylene blue pathogen inactivation on the integrity of immunoglobulin M and G",
"author": "Raster",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Transfus Med Hemother",
"key": "10.1016/S2213-2600(21)00545-2_bib36",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"article-title": "Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial",
"author": "Bégin",
"doi-asserted-by": "crossref",
"first-page": "2012",
"journal-title": "Nat Med",
"key": "10.1016/S2213-2600(21)00545-2_bib37",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3390/v13081594",
"article-title": "COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors",
"author": "Focosi",
"doi-asserted-by": "crossref",
"journal-title": "Viruses",
"key": "10.1016/S2213-2600(21)00545-2_bib38",
"volume": "13",
"year": "2021"
},
{
"article-title": "Photodynamic action of methylene blue on antipneumococcal serum",
"author": "Ross",
"first-page": "351",
"journal-title": "J Immunol",
"key": "10.1016/S2213-2600(21)00545-2_bib39",
"volume": "35",
"year": "1938"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021005452"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}