Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19
et al., Nature Communications, doi:10.1038/s41467-022-32551-2, ACTIV-2/A5401, NCT04427501, Aug 2022
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 317 outpatients in the USA showing faster viral load and inflammatory biomarker decline, but no significant differences in clinical outcomes.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 25.5% lower, RR 0.75, p = 0.60, treatment 6 of 159 (3.8%), control 8 of 158 (5.1%), NNT 78, combined.
|
|
risk of hospitalization, 52.1% lower, RR 0.48, p = 0.43, treatment 2 of 48 (4.2%), control 4 of 46 (8.7%), NNT 22, 7000mg, day 28.
|
|
risk of hospitalization, 0.9% higher, RR 1.01, p = 1.00, treatment 4 of 111 (3.6%), control 4 of 112 (3.6%), 700mg, day 28.
|
|
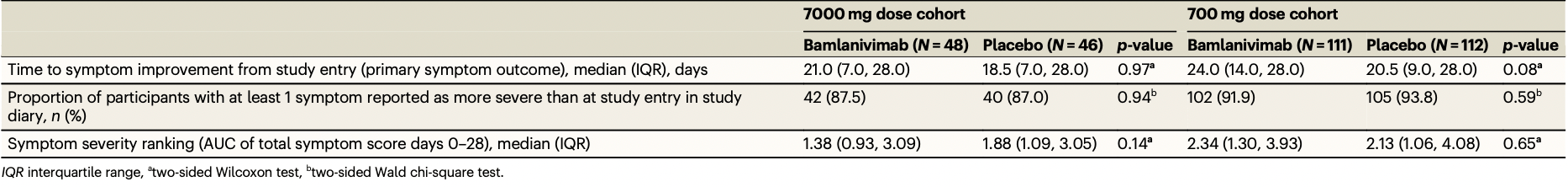
relative time to symptom improvement, 13.5% higher, relative time 1.14, p = 0.97, treatment 48, control 46, 7000mg, primary outcome.
|
|
relative time to symptom improvement, 17.1% higher, relative time 1.17, p = 0.08, treatment 111, control 112, 700mg, primary outcome.
|
|
risk of progression, 0.6% higher, RR 1.01, p = 1.00, treatment 42 of 48 (87.5%), control 40 of 46 (87.0%), at least one symptom more severe than baseline, 7000mg.
|
|
risk of progression, 2.0% lower, RR 0.98, p = 0.62, treatment 102 of 111 (91.9%), control 105 of 112 (93.8%), NNT 54, at least one symptom more severe than baseline, 700mg.
|
|
viral load, 25.6% lower, relative load 0.74, p = 0.002, treatment 48, control 46, 7000mg, day 3.
|
|
viral load, 35.3% lower, relative load 0.65, p = 0.07, treatment 111, control 112, 700mg, day 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Chew et al., 22 Aug 2022, Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 26 authors, study period 19 August, 2020 - 15 November, 2020, average treatment delay 6.0 days, trial NCT04427501 (history) (ACTIV-2/A5401).
Contact: kchew@mednet.ucla.edu.
Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19
Nature Communications, doi:10.1038/s41467-022-32551-2
Anti-SARS-CoV-2 monoclonal antibodies are mainstay COVID-19 therapeutics. Safety, antiviral, and clinical efficacy of bamlanivimab were evaluated in the randomized controlled trial ACTIV-2/A5401. Non-hospitalized adults were randomized 1:1 within 10 days of COVID-19 symptoms to bamlanivimab or blinded-placebo in two dose-cohorts (7000 mg, n = 94; 700 mg, n = 223). No differences in bamlanivimab vs placebo were observed in the primary outcomes: proportion with undetectable nasopharyngeal SARS-CoV-2 RNA at days 3, 7, 14, 21, and 28 (risk ratio = 0.82-1.05 for 7000 mg [p(overall) = 0.88] and 0.81-1.21 for 700 mg [p(overall) = 0.49]), time to symptom improvement (median 21 vs 18.5 days [p = 0.97], 7000 mg; 24 vs 20.5 days [p = 0.08], 700 mg), or grade 3+ adverse events. However, bamlanivimab was associated with lower day 3 nasopharyngeal viral levels and faster reductions in inflammatory markers and viral decay by modeling. This study provides evidence of faster reductions in nasopharyngeal SARS-CoV-2 RNA levels but not shorter symptom durations in non-hospitalized adults with early variants of SARS-CoV-2. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes Coronavirus disease 2019 (COVID-19), continues to exert an enormous global public health and economic toll, and in the U.S. case-fatality rates exceed estimates for the 1918 influenza pandemic 1 . Anti-SARS-CoV-2 monoclonal antibody (mAb)-based therapies have shown sufficient clinical efficacy to receive emergency
Author contributions
Competing interests Additional information Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41467-022-32551-2.
References
Aksamentov, Roemer, Hodcroft, Neher, Nextclade: clade assignment, mutation calling and quality control for viral genomes, J. Open Source Softw
Berg, Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity, J. Clin. Virol
Bernal, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N. Engl. J. Med
Chen, First-in-human study of Bamlanivimab in a randomized trial of hospitalized patients with COVID-19, Clin. Pharm. Ther
Chen, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N. Engl. J. Med
Chigutsa, O'brien, Ferguson-Sells, Long, Chien, Population pharmacokinetics and pharmacodynamics of the neutralizing antibodies Bamlanivimab and Etesevimab in patients with mild to moderate COVID-19 infection, Clin. Pharm. Ther
Degli-Angeli, Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance, J. Clin. Virol
Dougan, Bamlanivimab plus Etesevimab in mild or moderate Covid-19, N. Engl. J. Med
Fajnzylber, SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat. Commun
Gottlieb, Early Remdesivir to prevent progression to severe Covid-19 in outpatients, N. Engl. J. Med
Gottlieb, Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial, JAMA
Gupta, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody Sotrovimab, N. Engl. J. Med
Gupta, Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial, JAMA
Hammond, Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N. Engl. J. Med
Jacobs, Severe acute respiratory syndrome Coronavirus 2 Viremia is associated with Coronavirus Disease 2019 severity and predicts clinical outcomes, Clin. Infect. Dis
Johnson, Effect of Molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: A randomized, placebo-controlled trial, Ann. Intern. Med
Jones, The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates, Sci. Transl. Med
Merck, has received research funding to the institution from Merck Sharp & Dohme and is a consultant for Pardes Biosciences
North, Determining the incidence of asymptomatic SARS-CoV-2 among early recipients of COVID-19 vaccines (DIS-COVER-COVID-19): A prospective cohort study of healthcare workers before, during and after vaccination, Clin. Infect. Dis
Paquin-Proulx, Associations between antibody fc-mediated effector functions and long-term Sequelae in Ebola Virus survivors, Front. Immunol
Planas, Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Rambaut, A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology, Nat. Microbiol
Taubenberger, Kash, Morens, The 1918 influenza pandemic: 100 years of questions answered and unanswered, Sci. Transl. Med
Tzou, Tao, Pond, Shafer, Coronavirus Resistance Database (CoV-RDB): SARS-CoV-2 susceptibility to monoclonal antibodies, convalescent plasma, and plasma from vaccinated persons, PLoS One
Wang, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N. Engl. J. Med
Weinreich, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N. Engl. J. Med
DOI record:
{
"DOI": "10.1038/s41467-022-32551-2",
"ISSN": [
"2041-1723"
],
"URL": "http://dx.doi.org/10.1038/s41467-022-32551-2",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Anti-SARS-CoV-2 monoclonal antibodies are mainstay COVID-19 therapeutics. Safety, antiviral, and clinical efficacy of bamlanivimab were evaluated in the randomized controlled trial ACTIV-2/A5401. Non-hospitalized adults were randomized 1:1 within 10 days of COVID-19 symptoms to bamlanivimab or blinded-placebo in two dose-cohorts (7000 mg, <jats:italic>n</jats:italic> = 94; 700 mg, <jats:italic>n</jats:italic> = 223). No differences in bamlanivimab vs placebo were observed in the primary outcomes: proportion with undetectable nasopharyngeal SARS-CoV-2 RNA at days 3, 7, 14, 21, and 28 (risk ratio = 0.82–1.05 for 7000 mg [<jats:italic>p</jats:italic>(overall) = 0.88] and 0.81–1.21 for 700 mg [<jats:italic>p</jats:italic>(overall) = 0.49]), time to symptom improvement (median 21 vs 18.5 days [<jats:italic>p</jats:italic> = 0.97], 7000 mg; 24 vs 20.5 days [<jats:italic>p</jats:italic> = 0.08], 700 mg), or grade 3+ adverse events. However, bamlanivimab was associated with lower day 3 nasopharyngeal viral levels and faster reductions in inflammatory markers and viral decay by modeling. This study provides evidence of faster reductions in nasopharyngeal SARS-CoV-2 RNA levels but not shorter symptom durations in non-hospitalized adults with early variants of SARS-CoV-2.</jats:p>",
"alternative-id": [
"32551"
],
"article-number": "4931",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "4 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "4 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 August 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "K.W.C. has received research funding to the institution from Merck Sharp & Dohme and is a consultant for Pardes Biosciences. E.S.D. receives consulting fees from Gilead Sciences, Merck, and GSK/ViiV and research support through the institution from Gilead Sciences and GSK/ViiV. D.A.W. has received funding to the institution to support research and honoraria for advisory boards and consulting from Gilead Sciences. J.Z.L. has consulted for Abbvie. C.M. has received research funding to the institution from E.L. P.K., K.P., and A.N. are employees and shareholders of E.L. W.F. has received research funding to the institution from Ridgeback Biopharmaceuticals, served on adjudication committees for Janssen, Syneos, and consulted for Roche and Merck. J.J.E. is an ad hoc consultant to GSK/VIR, data monitoring committee (DMC) chair for Adagio Phase III studies. J.S.C. has consulted for Merck and Company. D.M.S. has consulted for the following companies Fluxergy, Kiadis, Linear Therapies, Matrix BioMed, Arena Pharmaceuticals, VxBiosciences, Model Medicines, Bayer Pharmaceuticals, Signant Health and Brio Clinical. All other authors (R.C., C.M., J.R., M.G., A.C.J., Y.L., M.C.C., R.D., V.B., R.M.R., A.S.P., C.V.F., and M.D.H.) report no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4865-4348",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chew",
"given": "Kara W.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5601-9112",
"affiliation": [],
"authenticated-orcid": false,
"family": "Moser",
"given": "Carlee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daar",
"given": "Eric S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7764-0212",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wohl",
"given": "David A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9914-9662",
"affiliation": [],
"authenticated-orcid": false,
"family": "Li",
"given": "Jonathan Z.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2709-0512",
"affiliation": [],
"authenticated-orcid": false,
"family": "Coombs",
"given": "Robert W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6307-4849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ritz",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giganti",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Javan",
"given": "Arzhang Cyrus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yijia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhary",
"given": "Manish C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deo",
"given": "Rinki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malvestutto",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klekotka",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Price",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nirula",
"given": "Ajay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fischer",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bala",
"given": "Veenu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3988-8241",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ribeiro",
"given": "Ruy M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2455-0002",
"affiliation": [],
"authenticated-orcid": false,
"family": "Perelson",
"given": "Alan S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3703-7849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fletcher",
"given": "Courtney V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4938-0644",
"affiliation": [],
"authenticated-orcid": false,
"family": "Eron",
"given": "Joseph J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4279-4737",
"affiliation": [],
"authenticated-orcid": false,
"family": "Currier",
"given": "Judith S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hughes",
"given": "Michael D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3603-1733",
"affiliation": [],
"authenticated-orcid": false,
"family": "Smith",
"given": "Davey M.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "ACTIV-2/A5401 Study Team",
"sequence": "additional"
}
],
"container-title": "Nature Communications",
"container-title-short": "Nat Commun",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
22
]
],
"date-time": "2022-08-22T15:05:35Z",
"timestamp": 1661180735000
},
"deposited": {
"date-parts": [
[
2022,
8,
22
]
],
"date-time": "2022-08-22T15:05:51Z",
"timestamp": 1661180751000
},
"funder": [
{
"DOI": "10.13039/100000060",
"award": [
"UM1AI068636",
"UM1AI068634",
"UM1AI106701",
"R01AI028433"
],
"doi-asserted-by": "publisher",
"name": "U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases"
},
{
"name": "U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases"
},
{
"name": "U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases"
},
{
"name": "U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases"
},
{
"DOI": "10.13039/100000052",
"award": [
"R01OD011095"
],
"doi-asserted-by": "publisher",
"name": "U.S. Department of Health & Human Services | NIH | NIH Office of the Director"
},
{
"DOI": "10.13039/100008902",
"award": [
"LDRD 20200743ER, 20200695ER, and 20210730ER"
],
"doi-asserted-by": "publisher",
"name": "DOE | LDRD | Los Alamos National Laboratory"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
23
]
],
"date-time": "2022-08-23T04:10:44Z",
"timestamp": 1661227844659
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
8,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
22
]
],
"date-time": "2022-08-22T00:00:00Z",
"timestamp": 1661126400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
22
]
],
"date-time": "2022-08-22T00:00:00Z",
"timestamp": 1661126400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41467-022-32551-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41467-022-32551-2",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41467-022-32551-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
8,
22
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1126/scitranslmed.aau5485",
"doi-asserted-by": "crossref",
"key": "32551_CR1",
"unstructured": "Taubenberger, J. K., Kash, J. C. & Morens, D. M. The 1918 influenza pandemic: 100 years of questions answered and unanswered. Sci. Transl. Med. 11, eaau5485 (2019)."
},
{
"DOI": "10.1056/NEJMoa2029849",
"author": "P Chen",
"doi-asserted-by": "publisher",
"first-page": "229",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR2",
"unstructured": "Chen, P. et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 384, 229–237 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "632",
"journal-title": "JAMA",
"key": "32551_CR3",
"unstructured": "Gottlieb, R. L. et al. Effect of Bamlanivimab as monotherapy or in combination with Etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 325, 632–644 (2021).",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR4",
"unstructured": "Weinreich, D. M. et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 384, 238–251 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR5",
"unstructured": "Gupta, A. et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody Sotrovimab. N. Engl. J. Med. 385, 1941–1950 (2021).",
"volume": "385",
"year": "2021"
},
{
"key": "32551_CR6",
"unstructured": "Jones, B. E. et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 13, eabf1906 (2021)."
},
{
"key": "32551_CR7",
"unstructured": "US FDA. Fact Sheet for Health Care Providers. Emergency Use Authorization (EUA) of Bamlanivimab, US Food and Drug Administration. 2021."
},
{
"DOI": "10.1038/s41586-021-03398-2",
"author": "P Wang",
"doi-asserted-by": "publisher",
"first-page": "130",
"journal-title": "Nature",
"key": "32551_CR8",
"unstructured": "Wang, P. et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593, 130–135 (2021).",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "276",
"journal-title": "Nature",
"key": "32551_CR9",
"unstructured": "Planas, D. et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596, 276–280 (2021).",
"volume": "596",
"year": "2021"
},
{
"key": "32551_CR10",
"unstructured": "Weinreich, D. M. et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N. Engl. J. Med. 385, (2021)."
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR11",
"unstructured": "Gottlieb, R. L. et al. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR12",
"unstructured": "Hammond, J. et al. Oral Nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR13",
"unstructured": "Jayk Bernal, A. et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1002/cpt.2420",
"author": "E Chigutsa",
"doi-asserted-by": "publisher",
"first-page": "1302",
"journal-title": "Clin. Pharm. Ther.",
"key": "32551_CR14",
"unstructured": "Chigutsa, E., O’Brien, L., Ferguson-Sells, L., Long, A. & Chien, J. Population pharmacokinetics and pharmacodynamics of the neutralizing antibodies Bamlanivimab and Etesevimab in patients with mild to moderate COVID-19 infection. Clin. Pharm. Ther. 110, 1302–1310 (2021).",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"author": "M Dougan",
"doi-asserted-by": "publisher",
"first-page": "1382",
"journal-title": "N. Engl. J. Med.",
"key": "32551_CR15",
"unstructured": "Dougan, M. et al. Bamlanivimab plus Etesevimab in mild or moderate Covid-19. N. Engl. J. Med. 385, 1382–1392 (2021).",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1236",
"journal-title": "JAMA",
"key": "32551_CR16",
"unstructured": "Gupta, A. et al. Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 327, 1236–1246 (2022).",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.7326/M22-0729",
"doi-asserted-by": "crossref",
"key": "32551_CR17",
"unstructured": "Johnson, M. G. et al. Effect of Molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: A randomized, placebo-controlled trial. Ann. Intern. Med. 175, 1126–1134 (2022)."
},
{
"DOI": "10.3389/fimmu.2021.682120",
"author": "D Paquin-Proulx",
"doi-asserted-by": "publisher",
"first-page": "682120",
"journal-title": "Front. Immunol.",
"key": "32551_CR18",
"unstructured": "Paquin-Proulx, D. et al. Associations between antibody fc-mediated effector functions and long-term Sequelae in Ebola Virus survivors. Front. Immunol. 12, 682120 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab686",
"author": "JL Jacobs",
"doi-asserted-by": "publisher",
"first-page": "1525",
"journal-title": "Clin. Infect. Dis.",
"key": "32551_CR19",
"unstructured": "Jacobs, J. L. et al. Severe acute respiratory syndrome Coronavirus 2 Viremia is associated with Coronavirus Disease 2019 severity and predicts clinical outcomes. Clin. Infect. Dis. 74, 1525–1533 (2022).",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"author": "J Fajnzylber",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Commun.",
"key": "32551_CR20",
"unstructured": "Fajnzylber, J. et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 11, 5493 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/cpt.2405",
"author": "P Chen",
"doi-asserted-by": "publisher",
"first-page": "1467",
"journal-title": "Clin. Pharm. Ther.",
"key": "32551_CR21",
"unstructured": "Chen, P. et al. First-in-human study of Bamlanivimab in a randomized trial of hospitalized patients with COVID-19. Clin. Pharm. Ther. 110, 1467–1477 (2021).",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2021.104945",
"author": "MG Berg",
"doi-asserted-by": "publisher",
"first-page": "104945",
"journal-title": "J. Clin. Virol.",
"key": "32551_CR22",
"unstructured": "Berg, M. G. et al. Development of the RealTime SARS-CoV-2 quantitative Laboratory Developed Test and correlation with viral culture as a measure of infectivity. J. Clin. Virol. 143, 104945 (2021).",
"volume": "143",
"year": "2021"
},
{
"key": "32551_CR23",
"unstructured": "Abbott Molecular Inc. Abbott RealTime SARS-CoV-2 Assay Emergency Use Authorization (EUA) Package Insert, Abbott. 2021."
},
{
"DOI": "10.1016/j.jcv.2020.104474",
"author": "E Degli-Angeli",
"doi-asserted-by": "publisher",
"first-page": "104474",
"journal-title": "J. Clin. Virol.",
"key": "32551_CR24",
"unstructured": "Degli-Angeli, E. et al. Validation and verification of the Abbott RealTime SARS-CoV-2 assay analytical and clinical performance. J. Clin. Virol. 129, 104474 (2020).",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab643",
"author": "CM North",
"doi-asserted-by": "publisher",
"first-page": "1275",
"journal-title": "Clin. Infect. Dis.",
"key": "32551_CR25",
"unstructured": "North, C. M. et al. Determining the incidence of asymptomatic SARS-CoV-2 among early recipients of COVID-19 vaccines (DISCOVER-COVID-19): A prospective cohort study of healthcare workers before, during and after vaccination. Clin. Infect. Dis. 74, 1275–1278 (2022).",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0261045",
"author": "PL Tzou",
"doi-asserted-by": "publisher",
"first-page": "e0261045",
"journal-title": "PLoS One",
"key": "32551_CR26",
"unstructured": "Tzou, P. L., Tao, K., Pond, S. L. K. & Shafer, R. W. Coronavirus Resistance Database (CoV-RDB): SARS-CoV-2 susceptibility to monoclonal antibodies, convalescent plasma, and plasma from vaccinated persons. PLoS One 17, e0261045 (2022).",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1038/s41564-020-0770-5",
"author": "A Rambaut",
"doi-asserted-by": "publisher",
"first-page": "1403",
"journal-title": "Nat. Microbiol.",
"key": "32551_CR27",
"unstructured": "Rambaut, A. et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 5, 1403–1407 (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.21105/joss.03773",
"doi-asserted-by": "crossref",
"key": "32551_CR28",
"unstructured": "Aksamentov, I., Roemer, C., Hodcroft, E. & Neher, R. Nextclade: clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 6, 3773 (2021)."
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41467-022-32551-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Physics and Astronomy",
"General Biochemistry, Genetics and Molecular Biology",
"General Chemistry",
"Multidisciplinary"
],
"subtitle": [],
"title": "Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
