Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2102685, NCT04427501, Oct 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
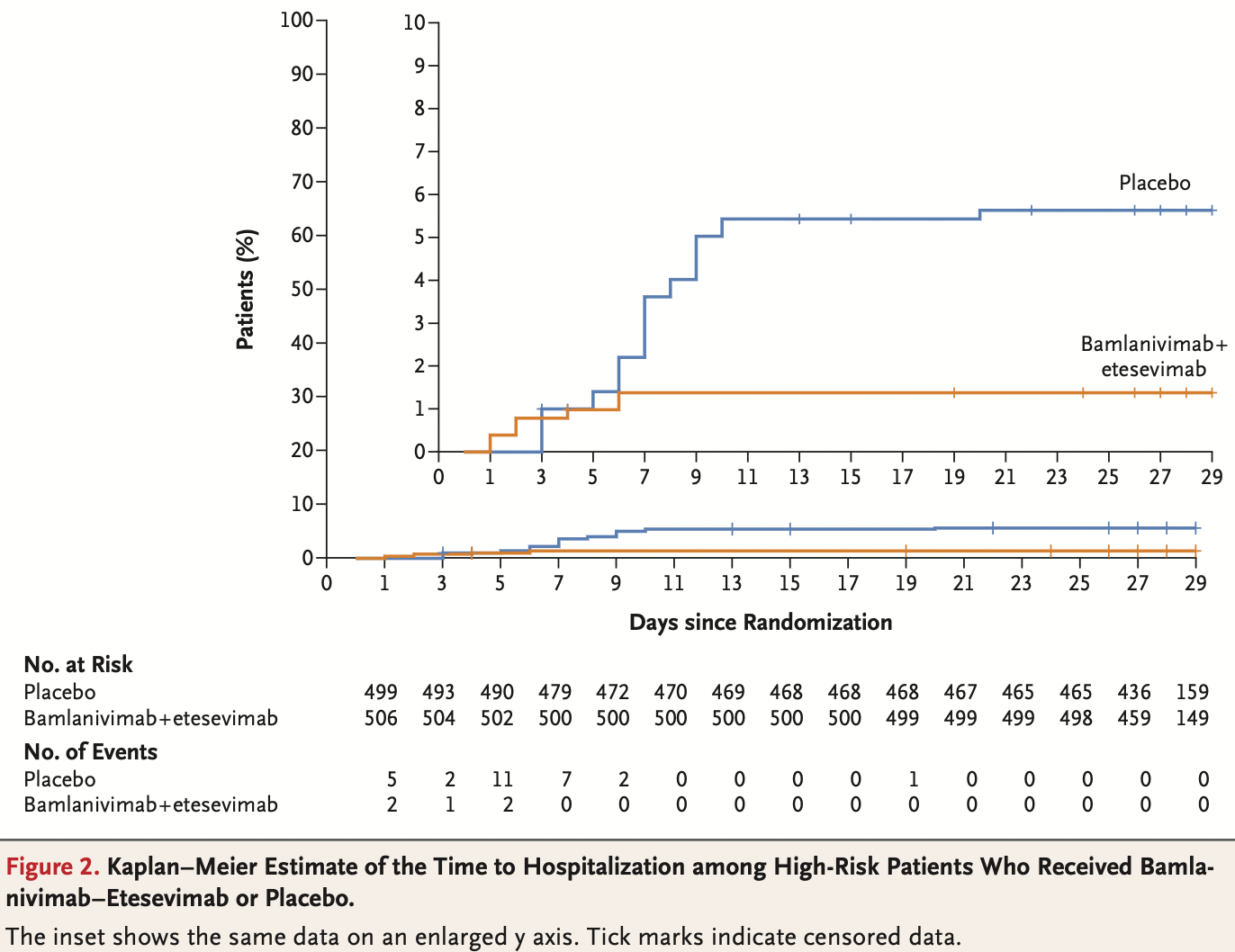
Results from the BLAZE-1 RCT of combined bamlanivimab/etesevimab, showing significantly lower mortality and combined mortality/hospitalization with treatment. NCT04427501 (history).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 94.7% lower, RR 0.05, p = 0.002, treatment 0 of 518 (0.0%), control 9 of 517 (1.7%), NNT 57, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), COVID-19 deaths.
|
|
risk of death/hospitalization, 69.5% lower, RR 0.30, p < 0.001, treatment 11 of 518 (2.1%), control 36 of 517 (7.0%), NNT 21, primary outcome.
|
|
recovery time, 11.1% lower, relative time 0.89, p = 0.007, treatment 518, control 517, sustained resolution of symptoms.
|
|
risk of no viral clearance, 66.6% lower, RR 0.33, p < 0.001, treatment 50 of 508 (9.8%), control 147 of 499 (29.5%), NNT 5.1, day 7, persistently high viral load.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Dougan et al., 7 Oct 2021, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 33 authors, study period 4 September, 2020 - 8 December, 2020, average treatment delay 4.0 days, trial NCT04427501 (history).
Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2102685
BACKGROUND Patients with underlying medical conditions are at increased risk for severe coronavirus disease 2019 (Covid-19). Whereas vaccine-derived immunity develops over time, neutralizing monoclonal-antibody treatment provides immediate, passive immunity and may limit disease progression and complications.
METHODS In this phase 3 trial, we randomly assigned, in a 1:1 ratio, a cohort of ambulatory patients with mild or moderate Covid-19 who were at high risk for progression to severe disease to receive a single intravenous infusion of either a neutralizing monoclonal-antibody combination agent (2800 mg of bamlanivimab and 2800 mg of etesevimab, administered together) or placebo within 3 days after a laboratory diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The primary outcome was the overall clinical status of the patients, defined as Covid-19-related hospitalization or death from any cause by day 29.
RESULTS A total of 1035 patients underwent randomization and received an infusion of bamlanivimab-etesevimab or placebo. The mean (±SD) age of the patients was 53.8±16.8 years, and 52.0% were adolescent girls or women. By day 29, a total of 11 of 518 patients (2.1%) in the bamlanivimab-etesevimab group had a Covid-19related hospitalization or death from any cause, as compared with 36 of 517 patients (7.0%) in the placebo group (absolute risk difference, −4.8 percentage points; 95% confidence interval [CI], −7.4 to −2.3; relative risk difference, 70%; P<0.001). No deaths occurred in the bamlanivimab-etesevimab group; in the placebo group, 10 deaths occurred, 9 of which were designated by the trial investigators as Covid-19-related. At day 7, a greater reduction from baseline in the log viral load was observed among patients who received bamlanivimab plus etesevimab than among those who received placebo (difference from placebo in the change from baseline, −1.20; 95% CI, −1.46 to −0.94; P<0.001).
CONCLUSIONS Among high-risk ambulatory patients, bamlanivimab plus etesevimab led to a lower incidence of Covid-19-related hospitalization and death than did placebo and accelerated the decline in the SARS-CoV-2 viral load. (Funded by Eli Lilly; BLAZE-1 ClinicalTrials.gov number, NCT04427501.
Appendix The authors' full names and academic degrees are as follows: Michael Dougan, M.D., Ph.D., Ajay Nirula, M.D., Ph.D., Masoud Azizad, M.D., Bharat Mocherla, M.D., Robert L. Gottlieb
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Benton, Wrobel, Xu, Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion, Nature
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med
Carfì, Bernabei, Landi, Persistent symptoms in patients after acute COVID-19, JAMA
Cates, Lucero-Obusan, Dahl, Risk for in-hospital complications associated with COVID-19 and influenza -Veterans Health Administration, United States, MMWR Morb Mortal Wkly Rep
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19, N Engl J Med
Chen, Liang, Jiang, Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China, Chest
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Chen, Zhang, Case, Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies, Nat Med
Chidambaram, Tun, Haque, Factors associated with disease severity and mortality among patients with COVID-19: a systematic review and metaanalysis, PLoS One
Del Rio, Collins, Malani, Longterm health consequences of COVID-19, JAMA
Ejaz, Alsrhani, Zafar, COVID-19 and comorbidities: deleterious impact on infected patients, J Infect Public Health
Garg, Kim, Whitaker, Hospitalization rates and characteristics of patients hospitalized with laboratoryconfirmed coronavirus disease 2019 -COVID-NET, 14 states, MMWR Morb Mortal Wkly Rep
Gosain, Abdou, Singh, Rana, Puzanov et al., COVID-19 and cancer: a comprehensive review, Curr Oncol Rep
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Halpin, Mcivor, Whyatt, Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation, J Med Virol
Jones, Brown-Augsburger, Corbett, LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection
Joyner, Senefeld, Klassen, Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, doi:10.1101/2020.08.12.20169359v1
Kim, Whitaker, 'halloran, Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 -COVID-NET, 14 states, MMWR Morb Mortal Wkly Rep
Li, Moore, Vasilieva, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature
Marston, Paules, Fauci, Monoclonal antibodies for emerging infectious diseases -borrowing from history, N Engl J Med
Piroth, Cottenet, Mariet, Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study, Lancet Respir Med
Roche, Roche provides an update on the phase III COVACTA Trial of Actemra/ RoActemra in hospitalised patients with severe COVID-19 associated pneumonia
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Shi, Shan, Duan, A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2, Nature
The, Group, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Xie, Tong, Guan, Du, Qiu, Clinical characteristics of patients who died of coronavirus disease 2019 in China, JAMA Netw Open
Zhou, Dejnirattisai, Supasa, Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera, Cell
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1056/nejmoa2102685",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/nejmoa2102685",
"alternative-id": [
"10.1056/NEJMoa2102685"
],
"author": [
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Dougan",
"given": "Michael",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Nirula",
"given": "Ajay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Azizad",
"given": "Masoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Mocherla",
"given": "Bharat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8376-8709",
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"authenticated-orcid": false,
"family": "Gottlieb",
"given": "Robert L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5330-1718",
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"authenticated-orcid": false,
"family": "Chen",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Hebert",
"given": "Corey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Perry",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Boscia",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Heller",
"given": "Barry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Morris",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Crystal",
"given": "Chad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Igbinadolor",
"given": "Awawu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Huhn",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Cardona",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Shawa",
"given": "Imad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Kumar",
"given": "Princy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Adams",
"given": "Andrew C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Van Naarden",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Custer",
"given": "Kenneth L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Durante",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Oakley",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Schade",
"given": "Andrew E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Holzer",
"given": "Timothy R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Ebert",
"given": "Philip J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Higgs",
"given": "Richard E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Kallewaard",
"given": "Nicole L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Sabo",
"given": "Janelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Patel",
"given": "Dipak R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Dabora",
"given": "Matan C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Klekotka",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Shen",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Massachusetts General Hospital and Harvard Medical School, Boston (M. Dougan); Eli Lilly (A.N., A.C.A., J.V.N., K.L.C., M. Durante, G.O., A.E.S., T.R.H., P.J.E., R.E.H., N.L.K., J.S., D.R.P., M.C.D., P. Klekotka, L.S., D.M.S.), and Franciscan Health (I.S.) — both in Indianapolis; Valley Clinical Trials–Northridge, Northridge (M.A.), the Department of Medicine, Women’s Guild Lung Institute, Cedars–Sinai Medical Center, Los Angeles (P.C.), and Long Beach Clinical Trials, Long Beach (B.H.) — all in..."
}
],
"family": "Skovronsky",
"given": "Daniel M.",
"sequence": "additional"
}
],
"container-title": [
"New England Journal of Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
14
]
],
"date-time": "2021-07-14T21:00:50Z",
"timestamp": 1626296450000
},
"deposited": {
"date-parts": [
[
2021,
10,
6
]
],
"date-time": "2021-10-06T21:12:50Z",
"timestamp": 1633554770000
},
"funder": [
{
"DOI": "10.13039/100004312",
"doi-asserted-by": "publisher",
"name": "Eli Lilly and Company"
}
],
"indexed": {
"date-parts": [
[
2022,
2,
14
]
],
"date-time": "2022-02-14T09:47:09Z",
"timestamp": 1644832029738
},
"is-referenced-by-count": 102,
"issn-type": [
{
"type": "print",
"value": "0028-4793"
},
{
"type": "electronic",
"value": "1533-4406"
}
],
"issue": "15",
"issued": {
"date-parts": [
[
2021,
10,
7
]
]
},
"journal-issue": {
"issue": "15",
"published-print": {
"date-parts": [
[
2021,
10,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
7
]
],
"date-time": "2021-10-07T00:00:00Z",
"timestamp": 1633564800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2102685",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "1382-1392",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
10,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
10,
7
]
]
},
"publisher": "Massachusetts Medical Society",
"reference-count": 27,
"references-count": 27,
"relation": {},
"score": 1,
"short-container-title": [
"N Engl J Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19"
],
"type": "journal-article",
"volume": "385"
}
