Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients with Mild-to-Moderate Coronavirus Disease 2019
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofac053, NCT04602000, Feb 2022
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
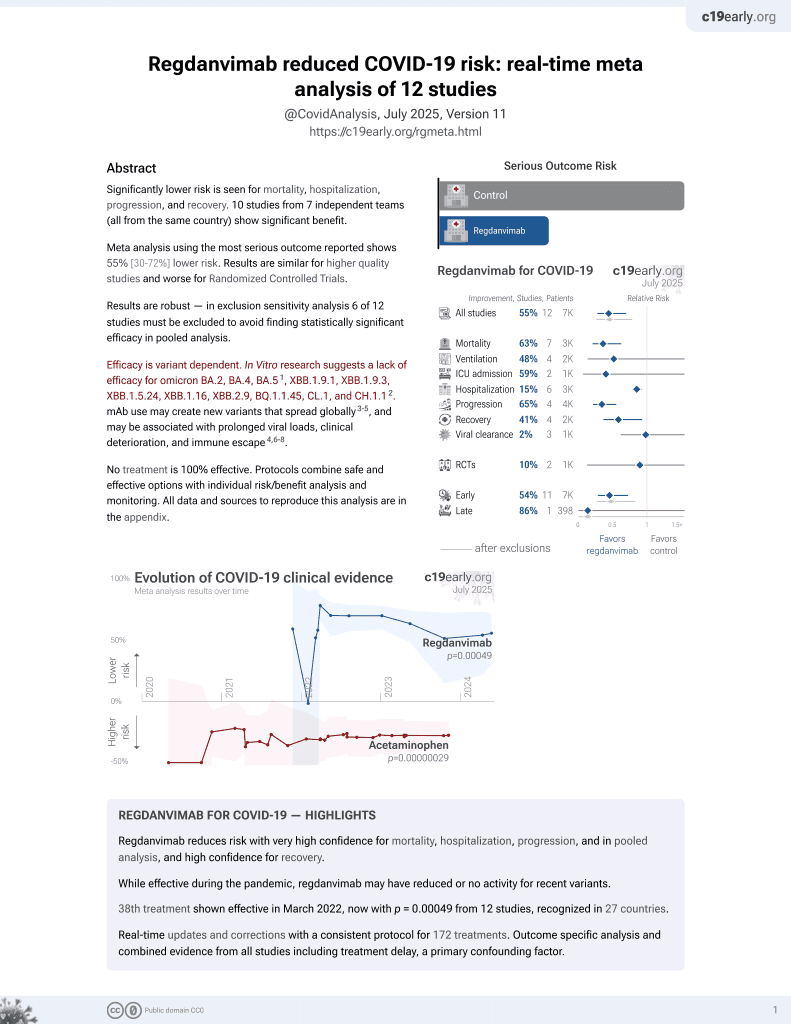
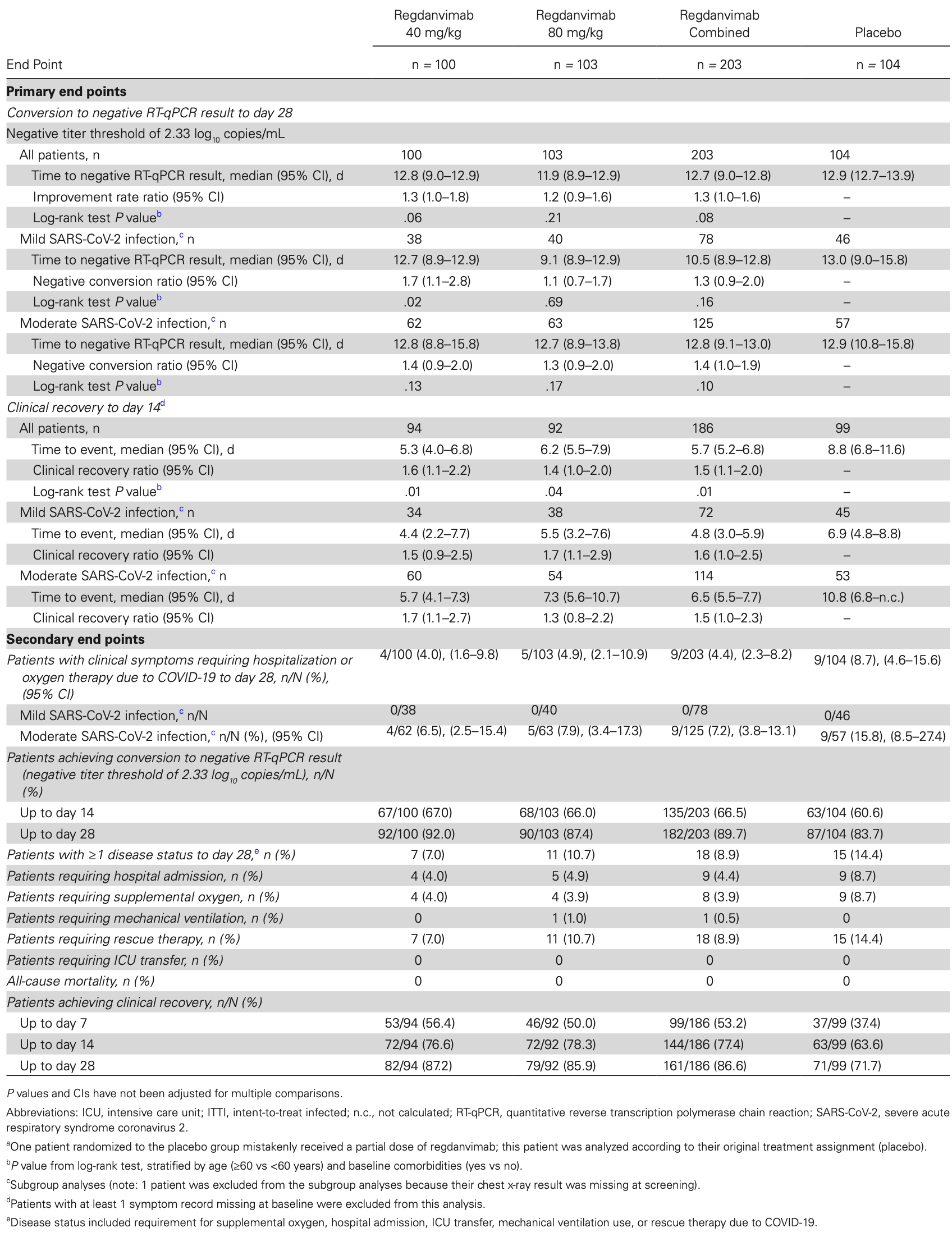
Phase 2 RCT with 307 outpatients with mild-moderate COVID-19, showing regdanvimab (monoclonal antibody) resulted in a minor decrease in time to negative PCR test (primary endpoint) compared to placebo, which was not statistically significant. Regdanvimab did significantly reduce time to clinical recovery by 3 days compared to placebo. A composite outcome of requiring hospitalization or oxygen therapy occurred in 4.4% of regdanvimab patients versus 8.7% placebo, with greater differences in moderate disease patients (7.2% vs 15.8% placebo). No safety issues were identified.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
|
risk of mechanical ventilation, 151.2% higher, RR 2.51, p = 1.00, treatment 1 of 203 (0.5%), control 0 of 104 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 54.5% lower, RR 0.46, p = 0.11, treatment 8 of 203 (3.9%), control 9 of 104 (8.7%), NNT 21.
|
|
risk of hospitalization, 48.8% lower, RR 0.51, p = 0.20, treatment 9 of 203 (4.4%), control 9 of 104 (8.7%), NNT 24.
|
|
composite outcome, 48.8% lower, RR 0.51, p = 0.20, treatment 9 of 203 (4.4%), control 9 of 104 (8.7%), NNT 24.
|
|
recovery time, 35.2% lower, relative time 0.65, p = 0.003, treatment mean 5.7 (±5.82) n=203, control mean 8.8 (±12.5) n=104.
|
|
time to viral-, 1.6% lower, relative time 0.98, p = 0.88, treatment mean 12.7 (±13.8) n=203, control mean 12.9 (±3.12) n=104.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Streinu-Cercel et al., 2 Feb 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 17 authors, average treatment delay 3.0 days, trial NCT04602000 (history).
Contact: helppl@gachon.ac.kr.
Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019
Open Forum Infectious Diseases, doi:10.1093/ofid/ofac053
Background. Regdanvimab (CT-P59) is a monoclonal antibody with neutralizing activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report on part 1 of a 2-part randomized, placebo-controlled, double-blind study for patients with mild-to-moderate coronavirus disease 2019 . Methods. Outpatients with mild-to-moderate COVID-19 received a single dose of regdanvimab 40 mg/kg (n = 100), regdanvimab 80 mg/kg (n = 103), or placebo (n = 104). The primary end points were time to negative conversion of SARS-CoV-2 from nasopharyngeal swab based on quantitative reverse transcription polymerase chain reaction (RT-qPCR) up to day 28 and time to clinical recovery up to day 14. Secondary end points included the proportion of patients requiring hospitalization, oxygen therapy, or mortality due to COVID-19. Results. Median (95% CI) time to negative conversion of RT-qPCR was 12.8 (9.0-12.9) days with regdanvimab 40 mg/kg, 11.9 (8.9-12.9) days with regdanvimab 80 mg/kg, and 12.9 (12.7-13.9) days with placebo. Median (95% CI) time to clinical recovery was 5.3 (4.0-6.8) days with regdanvimab 40 mg/kg, 6.2 (5.5-7.9) days with regdanvimab 80 mg/kg, and 8.8 (6.8-11.6) days with placebo. The proportion (95% CI) of patients requiring hospitalization or oxygen therapy was lower with regdanvimab 40 mg/kg (4.0% [1.6%-9.8%]) and regdanvimab 80 mg/kg (4.9% [2.1%-10.9%]) vs placebo (8.7% [4.6%-15.6%]). No serious treatment-emergent adverse events or deaths occurred. Conclusions. Regdanvimab showed a trend toward a minor decrease in time to negative conversion of RT-qPCR results compared with placebo and reduced the need for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19. Clinical trial registration. NCT04602000 and EudraCT 2020-003369-20.
Supplementary Data Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
Baden, Sahly, Essink, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med
Barek, Aziz, Islam, Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: a meta-analysis with 55 studies and 10014 cases, Heliyon
Blanco-Melo, Nilsson-Payant, Liu, Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell
Brookmeyer, Crowley, A confidence interval for the median survival time, Biometrics
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Clinicaltrials, A study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in preventing SARS-CoV-2 infection and COVID-19 in nursing home residents and staff (BLAZE-2) (NCT04497987)
Gisaid, Tracking of variants: VOC Delta, relative variant genome frequency per region
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Kim, Jang, Hong, Safety, virologic efficacy, and pharmacokinetics of CT-P59, a monoclonal antibody against SARS-CoV-2 spike receptor binding protein: two randomised phase 1 studies in healthy subjects and patients with mild SARS-CoV-2 infection, Clin Ther
Kim, Ryu, Lee, A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun
Lilly, Lilly's neutralizing antibody bamlanivimab (LY-CoV555) prevented COVID-19 at nursing homes in the BLAZE-2 trial, reducing risk by up to 80 percent for residents
Liu, Wang, Nair, Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike, Nature
Perkin, Heap, Crerar-Gilbert, Deaths in people from Black, Asian and minority ethnic communities from both COVID-19 and non-COVID causes in the first weeks of the pandemic in London: a hospital case note review, BMJ Open
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med
Sadoff, Gray, Vandebosch, Safety and efficacy of single-dose Ad26. COV2.S vaccine against Covid-19, N Engl J Med
Shi, Shan, Duan, A human neutralizing antibody targets the receptorbinding site of SARS-CoV-2, Nature
Stephenson, Gars, Sadoff, Immunogenicity of the Ad26.COV2.S vaccine for COVID-19, JAMA
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Wiley, Kubes, Cobb, Age, comorbid conditions, and racial disparities in COVID-19 outcomes, J Racial Ethn Health Disparities
Wilson, Probable inference, the law of succession, and statistical inference, J Am Stat Assoc
Wu, Wang, Shen, A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2, Science
Zost, Gilchuk, Case, Potently neutralizing and protective human antibodies against SARS-CoV-2, Nature
DOI record:
{
"DOI": "10.1093/ofid/ofac053",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofac053",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Regdanvimab (CT-P59) is a monoclonal antibody with neutralizing activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report on part 1 of a 2-part randomized, placebo-controlled, double-blind study for mild-to-moderate patients with coronavirus disease 2019 (COVID-19).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Outpatients with mild-to-moderate COVID-19 received a single dose of regdanvimab 40 mg/kg (n=100), regdanvimab 80 mg/kg (n=103), or placebo (n=104). Primary endpoints were time to negative conversion of SARS-CoV-2 from nasopharyngeal swab based on quantitative reverse transcription polymerase chain reaction (RT-qPCR) up to day 28 and time to clinical recovery up to day 14. Secondary endpoints included the proportion of patients requiring hospitalization, oxygen therapy, or mortality due to COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Median (95% confidence interval [CI]) time to negative conversion of RT-qPCR was 12.8 days (9.0–12.9) with regdanvimab 40 mg/kg, 11.9 days (8.9–12.9) with regdanvimab 80 mg/kg, and 12.9 days (12.7–13.9) with placebo. Median (95% CI) time to clinical recovery was 5.3 days (4.0–6.8) with regdanvimab 40 mg/kg, 6.2 days (5.5–7.9) with regdanvimab 80 mg/kg, and 8.8 days (6.8–11.6) with placebo. The proportion (95% CI) of patients requiring hospitalization or oxygen therapy was lower with regdanvimab 40 mg/kg (4.0% [1.6–9.8]) and regdanvimab 80 mg/kg (4.9% [2.1–10.9]) versus placebo (8.7% [4.6–15.6). No serious treatment-emergent adverse events or deaths occurred.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Regdanvimab showed a trend toward a minor decrease in time to negative conversion of RT-qPCR results compared with placebo and reduced the need for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof. Dr. Matei Balș”, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania"
}
],
"family": "Streinu-Cercel",
"given": "Anca",
"sequence": "first"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof. Dr. Matei Balș”, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania"
}
],
"family": "Săndulescu",
"given": "Oana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof. Dr. Matei Balș”, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania"
}
],
"family": "Preotescu",
"given": "Liliana-Lucia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Incheon Medical Center, Republic of Korea"
}
],
"family": "Kim",
"given": "Jin Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Chungnam National University School of Medicine, Daejeon, Republic of Korea"
}
],
"family": "Kim",
"given": "Yeon-Sook",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Chungnam National University School of Medicine, Daejeon, Republic of Korea"
}
],
"family": "Cheon",
"given": "Shinhye",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Incheon Medical Center, Republic of Korea"
}
],
"family": "Jang",
"given": "Young Rock",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Lee",
"given": "Sang Joon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Kim",
"given": "Sung Hyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Chang",
"given": "Ilsung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Suh",
"given": "Jee Hye",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Lee",
"given": "Seul Gi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Kim",
"given": "Mi Rim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Chung",
"given": "Da Rae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Celltrion, Inc., Incheon, Republic of Korea"
}
],
"family": "Kim",
"given": "Han Na",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases “Prof. Dr. Matei Balș”, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania"
}
],
"family": "Streinu-Cercel",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Gil Medical Center, Gachon University College of Medicine, Incheon, Republic of Korea"
}
],
"family": "Eom",
"given": "Joong Sik",
"sequence": "additional"
}
],
"container-title": [
"Open Forum Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T14:29:18Z",
"timestamp": 1643812158000
},
"deposited": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T14:29:19Z",
"timestamp": 1643812159000
},
"indexed": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T15:13:37Z",
"timestamp": 1643814817265
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2328-8957"
}
],
"issued": {
"date-parts": [
[
2022,
2,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T00:00:00Z",
"timestamp": 1643760000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac053/42367979/ofac053.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofac053/42367979/ofac053.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
2,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
2
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": [
"Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients with Mild-to-Moderate Coronavirus Disease 2019"
],
"type": "journal-article"
}