Real-World Evaluation of Bebtelovimab Effectiveness During the Period of COVID-19 Omicron Variants including BA.4/BA.5
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2023.04.396, Apr 2023
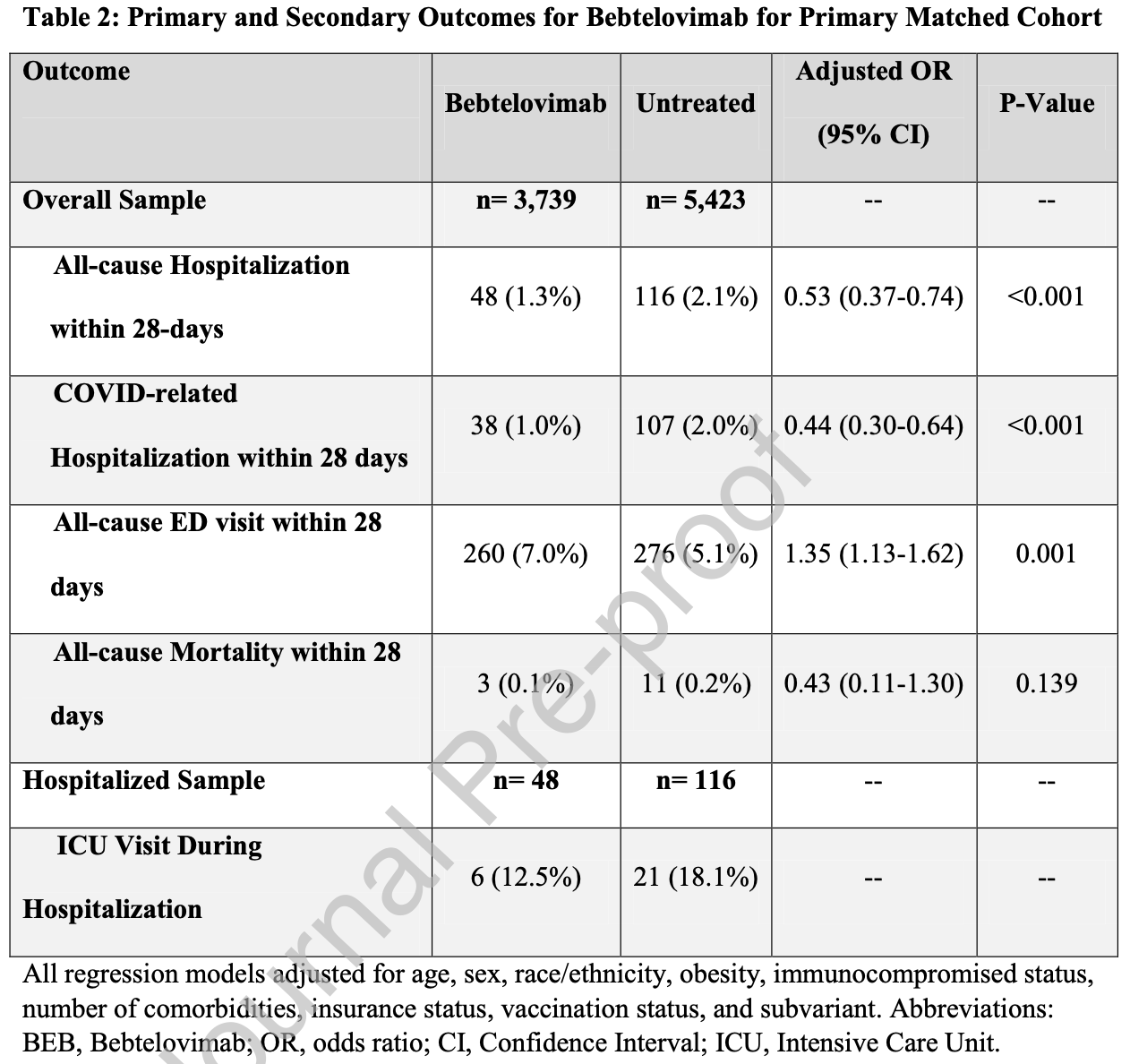
Retrospective 3,739 patients treated with bebteloviman in the USA and matched controls, showing lower mortality and hospitalization with treatment, but higher emergency department visits.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending bebtelovimab also recommended them, or
because the patient seeking out bebtelovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BQ.1.14, BA.5, BA.2.75, XBB5,6, XBB.1.5, XBB.1.9.16.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments7.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 57.0% lower, RR 0.43, p = 0.14, treatment 3 of 3,739 (0.1%), control 11 of 5,423 (0.2%), NNT 816, adjusted per study, odds ratio converted to relative risk, propensity score matching, multivariable.
|
|
risk of ICU admission, 58.6% lower, RR 0.41, p = 0.05, treatment 6 of 3,739 (0.2%), control 21 of 5,423 (0.4%), NNT 441.
|
|
risk of hospitalization, 55.5% lower, RR 0.44, p < 0.001, treatment 38 of 3,739 (1.0%), control 107 of 5,423 (2.0%), NNT 105, adjusted per study, odds ratio converted to relative risk, COVID-19, propensity score matching, multivariable.
|
|
risk of hospitalization, 46.5% lower, RR 0.54, p < 0.001, treatment 48 of 3,739 (1.3%), control 116 of 5,423 (2.1%), NNT 117, adjusted per study, odds ratio converted to relative risk, all cause, propensity score matching, multivariable.
|
|
risk of progression, 32.6% higher, RR 1.33, p = 0.001, treatment 260 of 3,739 (7.0%), control 275 of 5,423 (5.1%), adjusted per study, odds ratio converted to relative risk, ED visit, propensity score matching, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
5.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Molina et al., 16 Apr 2023, retrospective, USA, peer-reviewed, 11 authors, study period 6 April, 2022 - 11 October, 2022.
Contact: kyle.molina@cuanschutz.edu.
Real-World Evaluation of Bebtelovimab Effectiveness During the Period of COVID-19 Omicron Variants including BA.4/BA.5
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2023.04.396
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions AAG conceived and obtained funding for the study. KCM, VK LEB, NEC, NRA and AAG designed the study. VK, LEB, and NEC analysed the data. VK, LEB, TDB, NEC, DAM, and SR accessed and verified the data. KCM drafted the original version of the manuscript. All authors
Conflict of Interest Statement The authors report no conflicts of interest.
References
Aggarwal, Beaty, Bennett, Change in Effectiveness of Sotrovimab for Preventing Hospitalization and Mortality for At-risk COVID-19 Outpatients During an Omicron BA.1 and BA.1.1-Predominant Phase, International Journal of Infectious Diseases
Aggarwal, Beaty, Bennett, Real World Evidence of the Neutralizing Monoclonal Antibody Sotrovimab for Preventing Hospitalization and Mortality in COVID-19 Outpatients, J Infect Dis
Arora, Kempf, Nehlmeier, Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5, The Lancet Infectious Diseases
Austin, Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies, Pharm Stat
Dougan, Azizad, Chen, Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19, medRxiv, doi:10.1101/2022.03.10.22272100v1
Heinze, Schemper, A solution to the problem of separation in logistic regression, Statist Med
Heinze, logistf: Firth's Bias-Reduced Logistic Regression, R package version
Ho, Imai, King, Stuart, MatchIt: Nonparametric Preprocessing for Parametric Causal Inference, Journal of Statistical Software
Hoertel, Boulware, Sánchez-Rico, Burgun, Limosin, Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open
Lim, Tignanelli, Hoertel, Boulware, Usher, Prevalence of Medical Contraindications to Nirmatrelvir/Ritonavir in a Cohort of Hospitalized and Nonhospitalized Patients With COVID-19, Open Forum Infect Dis
Lin, Hung, Lai, Wang, Chen, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and meta-analysis of randomized controlled trials, J Med Virol
Mccreary, Kip, Collins, Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era, Open Forum Infectious Diseases
Nguyen, Collins, Spence, Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance, BMC Medical Research Methodology
Puhr, Heinze, Nold, Lusa, Geroldinger, Firth's logistic regression with rare events: accurate effect estimates and predictions?, Stat Med
Razonable, Horo, Hanson, Comparable Outcomes for Bebtelovimab and Ritonavir-Boosted Nirmatrelvir Treatment in High-Risk Patients With Coronavirus Disease-2019 During Severe Acute Respiratory Syndrome Coronavirus 2 BA.2 Omicron Epoch, The Journal of Infectious Diseases
Research, De, FDA updates Sotrovimab emergency use authorization
DOI record:
{
"DOI": "10.1016/j.ijid.2023.04.396",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2023.04.396",
"alternative-id": [
"S1201971223005258"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-World Evaluation of Bebtelovimab Effectiveness During the Period of COVID-19 Omicron Variants including BA.4/BA.5"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2023.04.396"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Molina",
"given": "Kyle C.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kennerley",
"given": "Victoria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8021-7872",
"affiliation": [],
"authenticated-orcid": false,
"family": "Beaty",
"given": "Laurel E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bennett",
"given": "Tellen D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carlson",
"given": "Nichole E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MayerIJ",
"given": "David A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peers",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2436-1367",
"affiliation": [],
"authenticated-orcid": false,
"family": "Russell",
"given": "Seth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3307-069X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wynia",
"given": "Matthew K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0922-8824",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aggarwal",
"given": "Neil R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
4,
16
]
],
"date-time": "2023-04-16T21:16:19Z",
"timestamp": 1681679779000
},
"deposited": {
"date-parts": [
[
2023,
4,
16
]
],
"date-time": "2023-04-16T21:16:20Z",
"timestamp": 1681679780000
},
"indexed": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T04:44:02Z",
"timestamp": 1681706642977
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
4
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T00:00:00Z",
"timestamp": 1680307200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2023,
4,
12
]
],
"date-time": "2023-04-12T00:00:00Z",
"timestamp": 1681257600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971223005258?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971223005258?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
4
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971223005258"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Real-World Evaluation of Bebtelovimab Effectiveness During the Period of COVID-19 Omicron Variants including BA.4/BA.5",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}