Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.08.005, CoV-Early, NCT04589949, Aug 2022
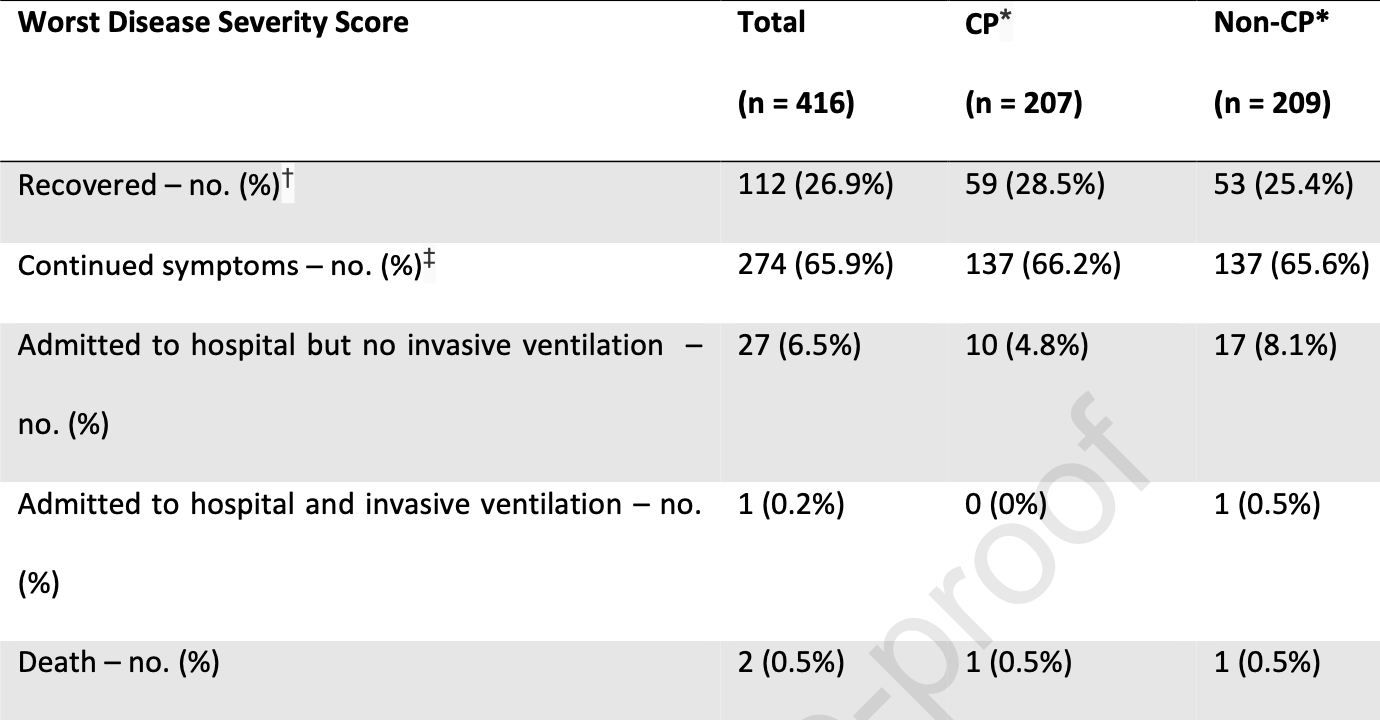
RCT 416 outpatients in the Netherlands, showing no significant difference with convalesent plasma treatment. Hospitalization was lower, and improved results were seen with ≤5 days of symptoms, without statistical significance.
|
risk of death, 1.0% higher, RR 1.01, p = 1.00, treatment 1 of 207 (0.5%), control 1 of 209 (0.5%), day 28.
|
|
risk of mechanical ventilation, 66.6% lower, RR 0.33, p = 1.00, treatment 0 of 207 (0.0%), control 1 of 209 (0.5%), NNT 209, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of progression, 14.0% lower, OR 0.86, p = 0.42, treatment 207, control 209, adjusted per study, improved severity score, RR approximated with OR.
|
|
risk of progression, 42.0% lower, OR 0.58, p = 0.06, treatment 123, control 103, adjusted per study, improved severity score, ≤5 days, RR approximated with OR.
|
|
risk of hospitalization, 39.0% lower, HR 0.61, p = 0.22, treatment 10 of 207 (4.8%), control 18 of 209 (8.6%), NNT 26, adjusted per study, day 28.
|
|
hospitalization time, 50.0% higher, relative time 1.50, p = 0.56, treatment 207, control 209.
|
|
risk of no recovery, 1.0% higher, RR 1.01, p = 0.92, treatment 137 of 207 (66.2%), control 137 of 209 (65.6%), continued COVID-19 symptoms, day 27.
|
|
recovery time, 8.3% higher, relative time 1.08, p = 0.99, treatment 207, control 209.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gharbharan et al., 23 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Netherlands, peer-reviewed, 59 authors, study period November 2020 - July 2021, average treatment delay 5.0 days, trial NCT04589949 (history) (CoV-Early).
Contact: b.rijnders@erasmusmc.nl, a.gharbharan@erasmusmc.nl.
Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: a randomized placebo-controlled trial
Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.08.005
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: We declare no conflicts of interest.
Acknowledgments: The
References
Alemany, Millat-Martinez, Corbacho-Monné, Malchair, Ouchi et al., High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial, The Lancet Respiratory Medicine
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, New England Journal of Medicine
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nature Communications
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, New England Journal of Medicine
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, New England Journal of Medicine
Huygens, Hofsink, Nijhof, Goorhuis, Kater et al., immunocompromised patients with COVID-19: a randomised, controlled, double-blind, phase 3 trial
Huygens, Munnink, Gharbharan, Koopmans, Rijnders, High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant, medRxiv
Korley, Durkalski-Mauldin, Yeatts, Schulman, Davenport et al., Early Convalescent Plasma for High-Risk Outpatients with Covid-19, New England Journal of Medicine
Kumar, Hu, Samson, Ferreira, Victoria et al., Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine, American Journal of Transplantation
Libster, Marc, Wappner, Coviello, Bianchi et al., Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults, New England Journal of Medicine
Malahe, Hoek, Dalm, Broers, Den Hoed et al., Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study, medRxiv
Petrilli, Jones, Yang, Rajagopalan, Donnell et al., Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ
Piechotta, Iannizzi, Chai, Valk, Kimber et al., Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database of Systematic Reviews
Rijnders, Huygens, Mitjà, Evidence-based dosing of convalescent plasma for COVID-19 in future trials, Clinical Microbiology and Infection
Rockwood, Theou, Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources, Can Geriatr J
Sullivan, Gebo, Shoham, Bloch, Lau et al., Early Outpatient Treatment for Covid-19 with Convalescent Plasma, New England Journal of Medicine
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, New England Journal of Medicine
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nature Medicine
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., None
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature
DOI record:
{
"DOI": "10.1016/j.cmi.2022.08.005",
"ISSN": [
"1198-743X"
],
"URL": "http://dx.doi.org/10.1016/j.cmi.2022.08.005",
"alternative-id": [
"S1198743X22004219"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Clinical Microbiology and Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.cmi.2022.08.005"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Published by Elsevier Ltd on behalf of European Society of Clinical Microbiology and Infectious Diseases."
}
],
"author": [
{
"affiliation": [],
"family": "Gharbharan",
"given": "Arvind",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jordans",
"given": "Carlijn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zwaginga",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Papageorgiou",
"given": "Grigorios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Geloven",
"given": "Nan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Wijngaarden",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "den Hollander",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karim",
"given": "Faiz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Leeuwen-Segarceanu",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soetekouw",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lammers",
"given": "Jolanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Postma",
"given": "Douwe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kampschreur",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Groeneveld",
"given": "Geert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swaneveld",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ellen van der Schoot",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Götz",
"given": "Hannelore",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haagmans",
"given": "Bart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koopmans",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bogers",
"given": "Susanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geurtsvankessel",
"given": "Corine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zwaginga",
"given": "Jaap Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rokx",
"given": "Casper",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rijnders",
"given": "Bart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katsikis",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muller",
"given": "Yvonne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miedema",
"given": "Jelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Struik",
"given": "Jelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rokx-Niemantsverdriet",
"given": "Lotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oud",
"given": "Josine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meier",
"given": "Romy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Zwet",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mooijaart",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albersen",
"given": "Arjan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vrielink",
"given": "Hans",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van de Watering",
"given": "Leo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hogema",
"given": "Boris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Etten",
"given": "Ronald",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Gammeren",
"given": "Adriaan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maas",
"given": "Nanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Ginneken",
"given": "Betty",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Verstijnen",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van den Berg – Rahman",
"given": "Juliette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hiddema",
"given": "Siepke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Elst",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reitsma",
"given": "Annette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molenkamp",
"given": "Karin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Band",
"given": "Caterina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Droog",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buitenhuis",
"given": "Lonneke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koster",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lukens",
"given": "Michaèl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scholtens",
"given": "Thea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van den Boomgaard",
"given": "Maartje",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vonk",
"given": "Machiel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Vonderen",
"given": "Marit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vrolijk",
"given": "Loes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reusken",
"given": "Chantal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reimerink",
"given": "Johan",
"sequence": "additional"
}
],
"container-title": "Clinical Microbiology and Infection",
"container-title-short": "Clinical Microbiology and Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalmicrobiologyandinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
23
]
],
"date-time": "2022-08-23T02:51:26Z",
"timestamp": 1661223086000
},
"deposited": {
"date-parts": [
[
2022,
8,
23
]
],
"date-time": "2022-08-23T02:51:39Z",
"timestamp": 1661223099000
},
"indexed": {
"date-parts": [
[
2022,
8,
23
]
],
"date-time": "2022-08-23T04:10:15Z",
"timestamp": 1661227815748
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22004219?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22004219?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib1",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"issue": "3",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib2",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "21",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib3",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib4",
"volume": "386",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib5",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "VanBlargan",
"doi-asserted-by": "crossref",
"journal-title": "Nature Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib6",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "crossref",
"key": "10.1016/j.cmi.2022.08.005_bib7",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. New England Journal of Medicine. 2022."
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "3189",
"issue": "1",
"journal-title": "Nature Communications",
"key": "10.1016/j.cmi.2022.08.005_bib8",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1966",
"article-title": "Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study",
"author": "Petrilli",
"doi-asserted-by": "crossref",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "10.1016/j.cmi.2022.08.005_bib9",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"issue": "7821",
"journal-title": "Nature",
"key": "10.1016/j.cmi.2022.08.005_bib10",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.5770/cgj.23.463",
"article-title": "Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources",
"author": "Rockwood",
"doi-asserted-by": "crossref",
"first-page": "210",
"issue": "3",
"journal-title": "Can Geriatr J",
"key": "10.1016/j.cmi.2022.08.005_bib11",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"issue": "7",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib12",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early Convalescent Plasma for High-Risk Outpatients with Covid-19",
"author": "Korley",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib13",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00545-2",
"article-title": "High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial",
"author": "Alemany",
"doi-asserted-by": "crossref",
"first-page": "278",
"issue": "3",
"journal-title": "The Lancet Respiratory Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib14",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2119657",
"article-title": "Early Outpatient Treatment for Covid-19 with Convalescent Plasma",
"author": "Sullivan",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.cmi.2022.08.005_bib15",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD013600.pub4",
"doi-asserted-by": "crossref",
"key": "10.1016/j.cmi.2022.08.005_bib16",
"unstructured": "Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews. 2021(5)."
},
{
"DOI": "10.1016/j.cmi.2022.01.026",
"article-title": "Evidence-based dosing of convalescent plasma for COVID-19 in future trials",
"author": "Rijnders",
"doi-asserted-by": "crossref",
"journal-title": "Clinical Microbiology and Infection",
"key": "10.1016/j.cmi.2022.08.005_bib17",
"year": "2022"
},
{
"article-title": "Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study",
"author": "Malahe",
"journal-title": "medRxiv",
"key": "10.1016/j.cmi.2022.08.005_bib18",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17020",
"article-title": "Neutralization against Omicron variant in transplant recipients after three doses of mRNA vaccine",
"author": "Kumar",
"doi-asserted-by": "crossref",
"journal-title": "American Journal of Transplantation",
"key": "10.1016/j.cmi.2022.08.005_bib19",
"year": "2022"
},
{
"article-title": "High incidence of sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant",
"author": "Huygens",
"journal-title": "medRxiv",
"key": "10.1016/j.cmi.2022.08.005_bib20",
"year": "2022"
},
{
"article-title": "SARS-CoV-2 hyperimmune globulin for severely immunocompromised patients with COVID-19: a randomised, controlled, double-blind, phase 3 trial",
"author": "Huygens",
"journal-title": "medRxiv",
"key": "10.1016/j.cmi.2022.08.005_bib21",
"year": "2022"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1198743X22004219"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}