Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial
et al., Nature Communications, doi:10.1038/s41467-021-22177-1, COVID-Lambda, NCT04331899, Mar 2021
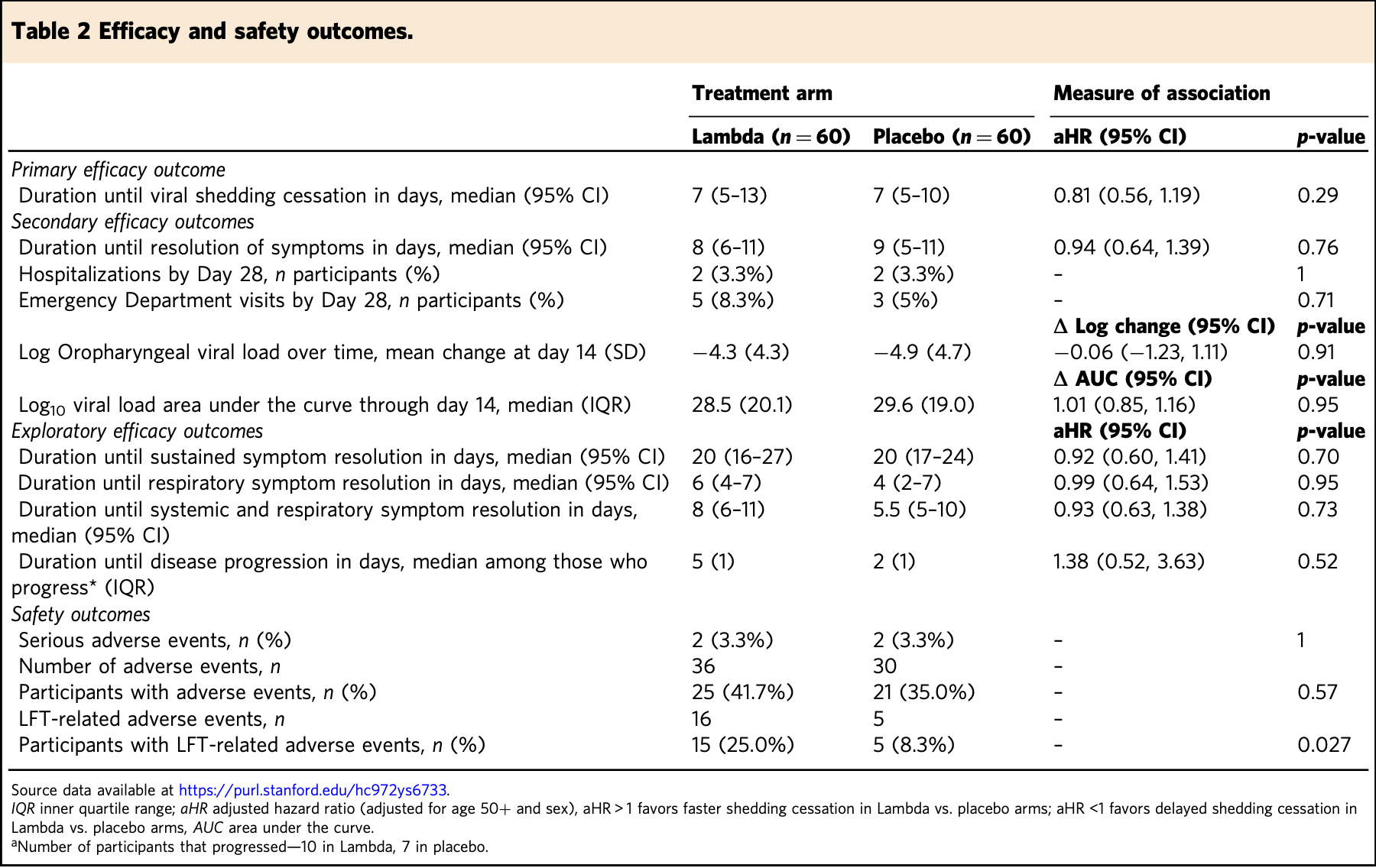
RCT 120 outpatients with mild/moderate COVID-19, showing no significant differences with peginterferon lambda-1a treatment. 180μg subcutaneous peginterferon lambda-1a.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, no change, RR 1.00, p = 1.00, treatment 2 of 60 (3.3%), control 2 of 60 (3.3%), day 28.
|
|
duration of symptoms, 6.4% higher, HR 1.06, p = 0.76, treatment 60, control 60, inverted to make HR<1 favor treatment.
|
|
relative change in viral load, 14.0% worse, RR 1.14, p = 0.91, treatment 60, control 60, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jagannathan et al., 30 Mar 2021, Single Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 27 authors, study period 25 April, 2020 - 17 July, 2020, average treatment delay 5.0 days, trial NCT04331899 (history) (COVID-Lambda).
Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial
Nature Communications, doi:10.1038/s41467-021-22177-1
Type III interferons have been touted as promising therapeutics in outpatients with coronavirus disease 2019 (COVID-19). We conducted a randomized, single-blind, placebocontrolled trial (NCT04331899) in 120 outpatients with mild to moderate COVID-19 to determine whether a single, 180 mcg subcutaneous dose of Peginterferon Lambda-1a (Lambda) within 72 hours of diagnosis could shorten the duration of viral shedding (primary endpoint) or symptoms (secondary endpoint). In both the 60 patients receiving Lambda and 60 receiving placebo, the median time to cessation of viral shedding was 7 days (hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.56 to 1.19). Symptoms resolved in 8 and 9 days in Lambda and placebo, respectively, and symptom duration did not differ significantly between groups (HR 0.94; 95% CI 0.64 to 1.39). Both Lambda and placebo were welltolerated, though liver transaminase elevations were more common in the Lambda vs. placebo arm (15/60 vs 5/60; p = 0.027). In this study, a single dose of subcutaneous Peginterferon Lambda-1a neither shortened the duration of SARS-CoV-2 viral shedding nor improved symptoms in outpatients with uncomplicated COVID-19.
Author contributions P.J., J.R.A., J.P., and U.S. designed the study and wrote the study protocol. C.L., H.H., J.P., V.B. developed study data instruments. C.H., I.C. provided study drug. P.J., J.R.A., H.B., K.B.J., S.K., C.R.d.V., O.Q., K.F., D.W., J.N., K.E., C.B., T.W., B.A.P., J.P., and U.S. collected data. C.H., I.C., Y.M., J.G., A.B., C.K. provided input on study procedures and data analysis. H.H., N.P., V.B., and M.D. prepared the statistical analysis plan and analyzed the data. All authors participated in data interpretation. K.B.J. and P.J. wrote the first draft and writing the manuscript and agreed on the decision to publish. There were no confidentiality agreements between the sponsors and authors.
Competing interests C.H. and I.C. are scientists at Eiger BioPharmaceuticals, Inc., which provided the Interferon Lambda used for this study. J.G. serves on the board of Eiger BioPharmaceuticals, Inc. C.H. and I.C. own stock and options of Eiger BioPharmaceuticals, Inc. J.G. has an equity interest in Eiger BioPharmaceuticals, Inc. J.G. and I.C. are inventors on a pending patent application relating to the use of interferon lambda for coronavirus. Eiger BioPharmaceuticals played no role in study design, conduct of the study, or analysis of the data. All other authors declare no competing interests.
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41467-021-22177-1...
References
Arunachalam, Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans, Science
Bastard, Autoantibodies against type I IFNs in patients with lifethreatening COVID-19, Science
Baud, Real estimates of mortality following COVID-19 infection, Lancet Infect. Dis
Broggi, Type III interferons disrupt the lung epithelial barrier upon viral recognition, Science
Bullard, Predicting infectious SARS-CoV-2 from diagnostic samples, Clin. Infect. Dis, doi:10.1093/cid/ciaa638
Center, Santa Clara County COVID-19 Demographics Dashboard
Chakraborty, Proinflammatory IgG Fc structures in patients with severe COVID-19, Nat. Immunol
Chan, Peginterferon lambda for the treatment of HBeAgpositive chronic hepatitis B: A randomized phase 2b study (LIRA-B), J. Hepatol
Chastain, Racial disproportionality in Covid Clinical Trials, N. Engl. J. Med
Chowkwanyun, Reed, Racial health disparities and Covid-19-caution and context, N. Engl. J. Med
Chu, Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19, Clin. Infect. Dis
Corman, Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Eur. Surveill
Dastan, Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial, Int. Immunopharmacol
Davidson, IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment, EMBO Mol. Med
Davoudi-Monfared, A Randomized Clinical Trial of the efficacy and safety of interferon beta-1a in treatment of severe COVID-19, Antimicrob. Agents Chemother
Dinnon, A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures, Nature
Egli, IL-28B is a key regulator of B-and T-cell vaccine responses against influenza, PLoS Pathog
Feld, Peginterferon-lambda for the treatment of COVID-19 in outpatients, medRxiv
Felgenhauer, Inhibition of SARS-CoV-2 by type I and type III interferons, J. Biol. Chem
Hadjadj, Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science
Hogan, Comparison of the Panther Fusion and a laboratorydeveloped test targeting the envelope gene for detection of SARS-CoV-2, J. Clin. Virol
Hogan, Sahoo, Pinsky, Sample pooling as a strategy to detect community transmission of SARS-CoV-2, JAMA
Hung, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet
Kotenko, IFN-lambdas, Curr. Opin. Immunol
Lazear, Schoggins, Diamond, Shared and distinct functions of Type I and Type III interferons, Immunity
Major, Type I and III interferons disrupt lung epithelial repair during recovery from viral infection, Science
Monk, Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, doubleblind, placebo-controlled, phase 2 trial, Lancet Respir. Med
Mordstein, Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections, J. Virol
Muir, A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection, J. Hepatol
Nair, Jacob, A simple practice guide for dose conversion between animals and human, J. Basic Clin. Pharm
O'brien, Weak induction of interferon expression by severe acute respiratory syndrome Coronavirus 2 Supports Clinical Trials of Interferonlambda to treat early Coronavirus Disease 2019, Clin. Infect. Dis
Pagliaccetti, Chu, Bolen, Kleinstein, Robek, Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities, Virology
Park, Iwasaki, Type I and Type III interferons-induction, signaling, evasion, and application to combat COVID-19, Cell Host Microbe
Price-Haywood, Burton, Fort, Seoane, Hospitalization and mortality among Black patients and White patients with Covid-19, N. Engl. J. Med
Prokunina-Olsson, COVID-19 and emerging viral infections: the case for interferon lambda, J. Exp. Med
Robek, Boyd, Chisari, Lambda interferon inhibits hepatitis B and C virus replication, J. Virol
Singanayagam, Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, Eur. Surveill
Sommereyns, Paul, Staeheli, Michiels, IFN-lambda (IFNlambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo, PLoS Pathog
Stokes, Coronavirus Disease 2019 Case Surveillance-United States, Morb. Mortal. Wkly Rep
Team, A Language and Environment for Statistical Computing (R Foundation for Statistical Computing
Weinreich, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N. Engl. J. Med
Yuen, SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists, Emerg. Microbes Infect
Zhang, Inborn errors of type I IFN immunity in patients with lifethreatening COVID-19, Science
Zhou, Interferon-alpha2b treatment for COVID-19, Front. Immunol
Zhou, Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases, J. Virol
DOI record:
{
"DOI": "10.1038/s41467-021-22177-1",
"ISSN": [
"2041-1723"
],
"URL": "http://dx.doi.org/10.1038/s41467-021-22177-1",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Type III interferons have been touted as promising therapeutics in outpatients with coronavirus disease 2019 (COVID-19). We conducted a randomized, single-blind, placebo-controlled trial (NCT04331899) in 120 outpatients with mild to moderate COVID-19 to determine whether a single, 180 mcg subcutaneous dose of Peginterferon Lambda-1a (Lambda) within 72 hours of diagnosis could shorten the duration of viral shedding (primary endpoint) or symptoms (secondary endpoint). In both the 60 patients receiving Lambda and 60 receiving placebo, the median time to cessation of viral shedding was 7 days (hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.56 to 1.19). Symptoms resolved in 8 and 9 days in Lambda and placebo, respectively, and symptom duration did not differ significantly between groups (HR 0.94; 95% CI 0.64 to 1.39). Both Lambda and placebo were well-tolerated, though liver transaminase elevations were more common in the Lambda vs. placebo arm (15/60 vs 5/60; p = 0.027). In this study, a single dose of subcutaneous Peginterferon Lambda-1a neither shortened the duration of SARS-CoV-2 viral shedding nor improved symptoms in outpatients with uncomplicated COVID-19.</jats:p>",
"alternative-id": [
"22177"
],
"article-number": "1967",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "22 December 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "3 March 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 March 2021"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "C.H. and I.C. are scientists at Eiger BioPharmaceuticals, Inc., which provided the Interferon Lambda used for this study. J.G. serves on the board of Eiger BioPharmaceuticals, Inc. C.H. and I.C. own stock and options of Eiger BioPharmaceuticals, Inc. J.G. has an equity interest in Eiger BioPharmaceuticals, Inc. J.G. and I.C. are inventors on a pending patent application relating to the use of interferon lambda for coronavirus. Eiger BioPharmaceuticals played no role in study design, conduct of the study, or analysis of the data. All other authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6305-758X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jagannathan",
"given": "Prasanna",
"sequence": "first"
},
{
"affiliation": [],
"family": "Andrews",
"given": "Jason R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonilla",
"given": "Hector",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0757-7959",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hedlin",
"given": "Haley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobson",
"given": "Karen B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balasubramanian",
"given": "Vidhya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Purington",
"given": "Natasha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamble",
"given": "Savita",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4667-5973",
"affiliation": [],
"authenticated-orcid": false,
"family": "de Vries",
"given": "Christiaan R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quintero",
"given": "Orlando",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7949-9531",
"affiliation": [],
"authenticated-orcid": false,
"family": "Feng",
"given": "Kent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ley",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Winslow",
"given": "Dean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Newberry",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Edwards",
"given": "Karlie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hislop",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choong",
"given": "Ingrid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maldonado",
"given": "Yvonne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Glenn",
"given": "Jeffrey",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8099-2975",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bhatt",
"given": "Ami",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6946-7627",
"affiliation": [],
"authenticated-orcid": false,
"family": "Blish",
"given": "Catherine",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3894-685X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Taia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khosla",
"given": "Chaitan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8751-4810",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pinsky",
"given": "Benjamin A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desai",
"given": "Manisha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7342-5366",
"affiliation": [],
"authenticated-orcid": false,
"family": "Parsonnet",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
}
],
"container-title": [
"Nature Communications"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
3,
30
]
],
"date-time": "2021-03-30T10:03:17Z",
"timestamp": 1617098597000
},
"deposited": {
"date-parts": [
[
2021,
12,
2
]
],
"date-time": "2021-12-02T07:22:26Z",
"timestamp": 1638429746000
},
"funder": [
{
"DOI": "10.13039/100006521",
"award": [
"Anonymous Donors"
],
"doi-asserted-by": "publisher",
"name": "SU | School of Medicine, Stanford University"
}
],
"indexed": {
"date-parts": [
[
2022,
3,
16
]
],
"date-time": "2022-03-16T22:05:18Z",
"timestamp": 1647468318511
},
"is-referenced-by-count": 38,
"issn-type": [
{
"type": "electronic",
"value": "2041-1723"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2021,
3,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
30
]
],
"date-time": "2021-03-30T00:00:00Z",
"timestamp": 1617062400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
30
]
],
"date-time": "2021-03-30T00:00:00Z",
"timestamp": 1617062400000
}
}
],
"link": [
{
"URL": "http://www.nature.com/articles/s41467-021-22177-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://www.nature.com/articles/s41467-021-22177-1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://www.nature.com/articles/s41467-021-22177-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
3,
30
]
]
},
"published-online": {
"date-parts": [
[
2021,
3,
30
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "22177_CR1",
"unstructured": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (Johns Hopkins University, 2021). https://coronavirus.jhu.edu/map.html."
},
{
"DOI": "10.1016/S1473-3099(20)30195-X",
"author": "D Baud",
"doi-asserted-by": "publisher",
"first-page": "773",
"journal-title": "Lancet Infect. Dis.",
"key": "22177_CR2",
"unstructured": "Baud, D. et al. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 20, 773 (2020).",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.05.008",
"author": "A Park",
"doi-asserted-by": "publisher",
"first-page": "870",
"journal-title": "Cell Host Microbe",
"key": "22177_CR3",
"unstructured": "Park, A. & Iwasaki, A. Type I and Type III interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.coi.2011.07.007",
"author": "SV Kotenko",
"doi-asserted-by": "publisher",
"first-page": "583",
"journal-title": "Curr. Opin. Immunol.",
"key": "22177_CR4",
"unstructured": "Kotenko, S. V. IFN-lambdas. Curr. Opin. Immunol. 23, 583–590 (2011).",
"volume": "23",
"year": "2011"
},
{
"DOI": "10.1128/JVI.02438-06",
"author": "Z Zhou",
"doi-asserted-by": "publisher",
"first-page": "7749",
"journal-title": "J. Virol.",
"key": "22177_CR5",
"unstructured": "Zhou, Z. et al. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81, 7749–7758 (2007).",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1016/j.immuni.2019.03.025",
"author": "HM Lazear",
"doi-asserted-by": "publisher",
"first-page": "907",
"journal-title": "Immunity",
"key": "22177_CR6",
"unstructured": "Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of Type I and Type III interferons. Immunity 50, 907–923 (2019).",
"volume": "50",
"year": "2019"
},
{
"DOI": "10.1080/22221751.2020.1780953",
"author": "CK Yuen",
"doi-asserted-by": "publisher",
"first-page": "1418",
"journal-title": "Emerg. Microbes Infect.",
"key": "22177_CR7",
"unstructured": "Yuen, C. K. et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 9, 1418–1428 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa410",
"author": "H Chu",
"doi-asserted-by": "publisher",
"first-page": "1400",
"journal-title": "Clin. Infect. Dis.",
"key": "22177_CR8",
"unstructured": "Chu, H. et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 71, 1400–1409 (2020).",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6261",
"author": "PS Arunachalam",
"doi-asserted-by": "publisher",
"first-page": "1210",
"journal-title": "Science",
"key": "22177_CR9",
"unstructured": "Arunachalam, P. S. et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/science.abc6027",
"author": "J Hadjadj",
"doi-asserted-by": "publisher",
"first-page": "718",
"journal-title": "Science",
"key": "22177_CR10",
"unstructured": "Hadjadj, J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1074/jbc.AC120.013788",
"author": "U Felgenhauer",
"doi-asserted-by": "publisher",
"first-page": "13958",
"journal-title": "J. Biol. Chem.",
"key": "22177_CR11",
"unstructured": "Felgenhauer, U. et al. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 295, 13958–13964 (2020).",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2708-8",
"author": "KH Dinnon",
"doi-asserted-by": "publisher",
"first-page": "560",
"journal-title": "Nature",
"key": "22177_CR12",
"unstructured": "Dinnon, K. H. et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 586, 560–566 (2020).",
"volume": "586",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01061",
"author": "Q Zhou",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "Front. Immunol.",
"key": "22177_CR13",
"unstructured": "Zhou, Q. et al. Interferon-alpha2b treatment for COVID-19. Front. Immunol. 11, 1061 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"author": "IF Hung",
"doi-asserted-by": "publisher",
"first-page": "1695",
"journal-title": "Lancet",
"key": "22177_CR14",
"unstructured": "Hung, I. F. et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704 (2020).",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30511-7",
"author": "PD Monk",
"doi-asserted-by": "publisher",
"first-page": "196",
"journal-title": "Lancet Respir. Med.",
"key": "22177_CR15",
"unstructured": "Monk, P. D. et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 9, 196–206 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2020.106688",
"author": "F Dastan",
"doi-asserted-by": "publisher",
"first-page": "106688",
"journal-title": "Int. Immunopharmacol.",
"key": "22177_CR16",
"unstructured": "Dastan, F. et al. Subcutaneous administration of interferon beta-1a for COVID-19: a non-controlled prospective trial. Int. Immunopharmacol. 85, 106688 (2020).",
"volume": "85",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01061-20",
"author": "E Davoudi-Monfared",
"doi-asserted-by": "publisher",
"first-page": "e01061",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "22177_CR17",
"unstructured": "Davoudi-Monfared, E. et al. A Randomized Clinical Trial of the efficacy and safety of interferon beta-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 64, e01061 (2020).",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00272-10",
"author": "M Mordstein",
"doi-asserted-by": "publisher",
"first-page": "5670",
"journal-title": "J. Virol.",
"key": "22177_CR18",
"unstructured": "Mordstein, M. et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 84, 5670–5677 (2010).",
"volume": "84",
"year": "2010"
},
{
"DOI": "10.1371/journal.ppat.1000017",
"author": "C Sommereyns",
"doi-asserted-by": "publisher",
"first-page": "e1000017",
"journal-title": "PLoS Pathog.",
"key": "22177_CR19",
"unstructured": "Sommereyns, C., Paul, S., Staeheli, P. & Michiels, T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4, e1000017 (2008).",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.1016/j.virol.2010.02.022",
"author": "NE Pagliaccetti",
"doi-asserted-by": "publisher",
"first-page": "197",
"journal-title": "Virology",
"key": "22177_CR20",
"unstructured": "Pagliaccetti, N. E., Chu, E. N., Bolen, C. R., Kleinstein, S. H. & Robek, M. D. Lambda and alpha interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology 401, 197–206 (2010).",
"volume": "401",
"year": "2010"
},
{
"DOI": "10.1128/JVI.79.6.3851-3854.2005",
"author": "MD Robek",
"doi-asserted-by": "publisher",
"first-page": "3851",
"journal-title": "J. Virol.",
"key": "22177_CR21",
"unstructured": "Robek, M. D., Boyd, B. S. & Chisari, F. V. Lambda interferon inhibits hepatitis B and C virus replication. J. Virol. 79, 3851–3854 (2005).",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.15252/emmm.201606413",
"author": "S Davidson",
"doi-asserted-by": "publisher",
"first-page": "1099",
"journal-title": "EMBO Mol. Med.",
"key": "22177_CR22",
"unstructured": "Davidson, S. et al. IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment. EMBO Mol. Med. 8, 1099–1112 (2016).",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1016/j.jhep.2014.07.022",
"author": "AJ Muir",
"doi-asserted-by": "publisher",
"first-page": "1238",
"journal-title": "J. Hepatol.",
"key": "22177_CR23",
"unstructured": "Muir, A. J. et al. A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J. Hepatol. 61, 1238–1246 (2014).",
"volume": "61",
"year": "2014"
},
{
"DOI": "10.1093/cid/ciaa453",
"author": "TR O'Brien",
"doi-asserted-by": "publisher",
"first-page": "1410",
"journal-title": "Clin. Infect. Dis.",
"key": "22177_CR24",
"unstructured": "O'Brien, T. R. et al. Weak induction of interferon expression by severe acute respiratory syndrome Coronavirus 2 Supports Clinical Trials of Interferon-lambda to treat early Coronavirus Disease 2019. Clin. Infect. Dis. 71, 1410–1412 (2020).",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1084/jem.20200653",
"author": "L Prokunina-Olsson",
"doi-asserted-by": "publisher",
"first-page": "e20200653",
"journal-title": "J. Exp. Med.",
"key": "22177_CR25",
"unstructured": "Prokunina-Olsson, L. et al. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 217, e20200653 (2020).",
"volume": "217",
"year": "2020"
},
{
"key": "22177_CR26",
"unstructured": "Centers for Disease Control and Prevention. CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel instructions for use (effective February 4, 2020). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html."
},
{
"DOI": "10.1038/s41590-020-00828-7",
"author": "S Chakraborty",
"doi-asserted-by": "publisher",
"first-page": "67",
"journal-title": "Nat. Immunol.",
"key": "22177_CR27",
"unstructured": "Chakraborty, S. et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 22, 67–73 (2020).",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1016/j.jhep.2015.12.018",
"author": "HLY Chan",
"doi-asserted-by": "publisher",
"first-page": "1011",
"journal-title": "J. Hepatol.",
"key": "22177_CR28",
"unstructured": "Chan, H. L. Y. et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B). J. Hepatol. 64, 1011–1019 (2016).",
"volume": "64",
"year": "2016"
},
{
"key": "22177_CR29",
"unstructured": "Eiger_BioPharmaceuticals. Investigators Brochure version 3 11, May 2018 (Eiger_BioPharmaceuticals, 2018)."
},
{
"DOI": "10.4103/0976-0105.177703",
"author": "AB Nair",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "J. Basic Clin. Pharm.",
"key": "22177_CR30",
"unstructured": "Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016).",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1126/science.abc2061",
"author": "J Major",
"doi-asserted-by": "publisher",
"first-page": "712",
"journal-title": "Science",
"key": "22177_CR31",
"unstructured": "Major, J. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369, 712–717 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1126/science.abc3545",
"author": "A Broggi",
"doi-asserted-by": "publisher",
"first-page": "706",
"journal-title": "Science",
"key": "22177_CR32",
"unstructured": "Broggi, A. et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 369, 706–712 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1004556",
"author": "A Egli",
"doi-asserted-by": "publisher",
"first-page": "e1004556",
"journal-title": "PLoS Pathog.",
"key": "22177_CR33",
"unstructured": "Egli, A. et al. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog. 10, e1004556 (2014).",
"volume": "10",
"year": "2014"
},
{
"key": "22177_CR34",
"unstructured": "Feld, J. J. et al. Peginterferon-lambda for the treatment of COVID-19 in outpatients. medRxiv, 2020.2011.2009.20228098 (2020)."
},
{
"DOI": "10.1126/science.abd4570",
"author": "Q Zhang",
"doi-asserted-by": "publisher",
"first-page": "eabd4570",
"journal-title": "Science",
"key": "22177_CR35",
"unstructured": "Zhang, Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020).",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4585",
"author": "P Bastard",
"doi-asserted-by": "publisher",
"first-page": "eabd4585",
"journal-title": "Science",
"key": "22177_CR36",
"unstructured": "Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020).",
"volume": "370",
"year": "2020"
},
{
"key": "22177_CR37",
"unstructured": "Center, C.o.S.C.E.O. Santa Clara County COVID-19 Demographics Dashboard, Vol. 2020 (Center, C.o.S.C.E.O)."
},
{
"DOI": "10.1056/NEJMsa2011686",
"author": "EG Price-Haywood",
"doi-asserted-by": "publisher",
"first-page": "2534",
"journal-title": "N. Engl. J. Med.",
"key": "22177_CR38",
"unstructured": "Price-Haywood, E. G., Burton, J., Fort, D. & Seoane, L. Hospitalization and mortality among Black patients and White patients with Covid-19. N. Engl. J. Med. 382, 2534–2543 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6924e2",
"author": "EK Stokes",
"doi-asserted-by": "publisher",
"first-page": "759",
"journal-title": "Morb. Mortal. Wkly Rep.",
"key": "22177_CR39",
"unstructured": "Stokes, E. K. et al. Coronavirus Disease 2019 Case Surveillance— United States, January 22–May 30, 2020. Morb. Mortal. Wkly Rep. 69, 759–765 (2020).",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2021971",
"author": "DB Chastain",
"doi-asserted-by": "publisher",
"first-page": "e59",
"journal-title": "N. Engl. J. Med.",
"key": "22177_CR40",
"unstructured": "Chastain, D. B. et al. Racial disproportionality in Covid Clinical Trials. N. Engl. J. Med. 383, e59 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2012910",
"author": "M Chowkwanyun",
"doi-asserted-by": "publisher",
"first-page": "201",
"journal-title": "N. Engl. J. Med",
"key": "22177_CR41",
"unstructured": "Chowkwanyun, M. & Reed, A. L. Jr. Racial health disparities and Covid-19—caution and context. N. Engl. J. Med 383, 201–203 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N. Engl. J. Med.",
"key": "22177_CR42",
"unstructured": "Weinreich, D. M. et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 384, 238–251 (2020).",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa638",
"doi-asserted-by": "publisher",
"key": "22177_CR43",
"unstructured": "Bullard, J. et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. ciaa638 https://doi.org/10.1093/cid/ciaa638 (2020). Epub ahead of print."
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.32.2001483",
"author": "A Singanayagam",
"doi-asserted-by": "publisher",
"first-page": "2001483",
"journal-title": "Eur. Surveill.",
"key": "22177_CR44",
"unstructured": "Singanayagam, A. et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eur. Surveill. 25, 2001483 (2020).",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5445",
"author": "CA Hogan",
"doi-asserted-by": "publisher",
"first-page": "1967",
"journal-title": "JAMA",
"key": "22177_CR45",
"unstructured": "Hogan, C. A., Sahoo, M. K. & Pinsky, B. A. Sample pooling as a strategy to detect community transmission of SARS-CoV-2. JAMA 323, 1967–1969 (2020).",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104383",
"author": "CA Hogan",
"doi-asserted-by": "publisher",
"first-page": "104383",
"journal-title": "J. Clin. Virol.",
"key": "22177_CR46",
"unstructured": "Hogan, C. A. et al. Comparison of the Panther Fusion and a laboratory-developed test targeting the envelope gene for detection of SARS-CoV-2. J. Clin. Virol. 127, 104383 (2020).",
"volume": "127",
"year": "2020"
},
{
"author": "VM Corman",
"first-page": "2000045",
"journal-title": "Eur. Surveill.",
"key": "22177_CR47",
"unstructured": "Corman, V. M. et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eur. Surveill. 25, 2000045 (2020).",
"volume": "25",
"year": "2020"
},
{
"key": "22177_CR48",
"unstructured": "Team, R.C. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019)."
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"score": 1,
"short-container-title": [
"Nat Commun"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Physics and Astronomy",
"General Biochemistry, Genetics and Molecular Biology",
"General Chemistry"
],
"subtitle": [],
"title": [
"Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}