Early Treatment with Pegylated Interferon Lambda for Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2209760, TOGETHER, NCT04727424, Feb 2023
The TOGETHER trial has extreme COI, impossible data, blinding failure, randomization failure, uncorrected errors, and many protocol violations. Authors do not respond to these issues and they have refused to release the data as promised. Some issues may apply only to specific arms.
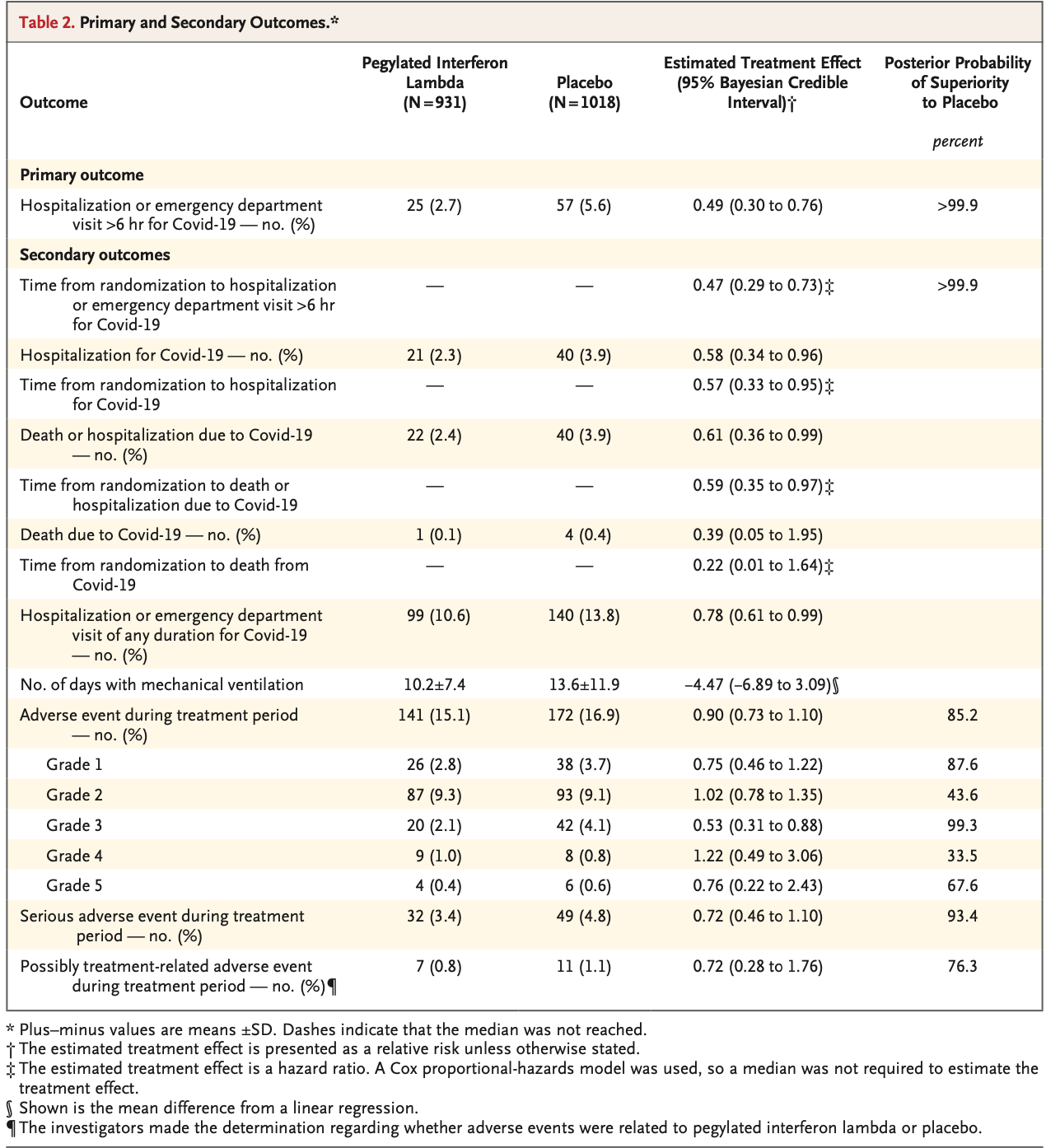
High-risk outpatient RCT with 931 peginterferon lambda patients and 1,018 control patients, showing significantly lower hospitalization/ER visits with treatment. Single subcutaneous injection.
There were 85/931 and 286/1018 patients for which baseline SARS-CoV-2 status was unknown, p = 1.4e-27 (about 1 in 704 septillion).
The most frequent risk factors were more common in the placebo group, for example obesity 39.1% control vs. 34.5% treatment, p = 0.04.
Authors claim patients were unaware or the randomization assignments, however some patients received oral placebo in a trial of a treatment requiring subcutaneous injection.
The numbers in Table 1 and Table S1 do not match, e.g., the text and Table 1 indicate 931 ITT interferon patients, while Table S1 shows 916.
All deaths in the placebo arm were attributed to COVID-19, while only 50% were in the interferon arm. One placebo death is listed as both due to COVID-19 and due to acute myeloid leukemia (Table S6).
See also1.
The TOGETHER trial has extreme COI, impossible data,
blinding failure, randomization failure, uncorrected errors, and many
protocol violations. Authors do not respond to these issues and they
have refused to release the data as promised. Some issues may apply only
to specific arms. For more details see2-6.
|
risk of death, 27.1% lower, RR 0.73, p = 0.76, treatment 4 of 931 (0.4%), control 6 of 1,018 (0.6%), NNT 626, all-cause, Table S6.
|
|
risk of death, 61.0% lower, RR 0.39, p = 0.32, treatment 1 of 931 (0.1%), control 4 of 1,018 (0.4%), adjusted per study, attributed to COVID, day 28.
|
|
risk of hospitalization, 42.0% lower, RR 0.58, p = 0.04, treatment 21 of 931 (2.3%), control 40 of 1,018 (3.9%), NNT 60, adjusted per study, day 28.
|
|
hospitalization or ER >6hrs, 51.0% lower, RR 0.49, p = 0.003, treatment 25 of 931 (2.7%), control 57 of 1,018 (5.6%), NNT 34, adjusted per study, day 28, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Kelleni, M., Peg-interferon Lambda Single Dose Treatment for COVID-19: A Call to Avoid another Hydroxychloroquine Fiasco, Center for Open Science, doi:10.31219/osf.io/5xd6q.
2.
Reis et al., Effect of Early Treatment with Ivermectin among Patients with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2115869.
3.
Reis (B) et al., RETRACTED: Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial, The Lancet Regional Health - Americas, doi:10.1016/j.lana.2021.100142.
4.
Reis (C) et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, The Lancet Global Health, doi:10.1016/S2214-109X(21)00448-4.
Reis et al., 9 Feb 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, 41 authors, study period 24 June, 2021 - 7 February, 2022, trial NCT04727424 (history) (TOGETHER).
Early Treatment with Pegylated Interferon Lambda for Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2209760
BACKGROUND The efficacy of a single dose of pegylated interferon lambda in preventing clinical events among outpatients with acute symptomatic coronavirus disease 2019 (Covid-19) is unclear.
METHODS We conducted a randomized, controlled, adaptive platform trial involving predominantly vaccinated adults with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brazil and Canada. Outpatients who presented with an acute clinical condition consistent with Covid-19 within 7 days after the onset of symptoms received either pegylated interferon lambda (single subcutaneous injection, 180 μg) or placebo (single injection or oral). The primary composite outcome was hospitalization (or transfer to a tertiary hospital) or an emergency department visit (observation for >6 hours) due to Covid-19 within 28 days after randomization.
RESULTS A total of 933 patients were assigned to receive pegylated interferon lambda (2 were subsequently excluded owing to protocol deviations) and 1018 were assigned to receive placebo. Overall, 83% of the patients had been vaccinated, and during the trial, multiple SARS-CoV-2 variants had emerged. A total of 25 of 931 patients (2.7%) in the interferon group had a primary-outcome event, as compared with 57 of 1018 (5.6%) in the placebo group, a difference of 51% (relative risk, 0.49; 95% Bayesian credible interval, 0.30 to 0.76; posterior probability of superiority to placebo, >99.9%). Results were generally consistent in analyses of secondary outcomes, including time to hospitalization for Covid-19 (hazard ratio, 0.57; 95% Bayesian credible interval, 0.33 to 0.95) and Covid-19-related hospitalization or death (hazard ratio, 0.59; 95% Bayesian credible interval, 0.35 to 0.97). The effects were consistent across dominant variants and independent of vaccination status. Among patients with a high viral load at baseline, those who received pegylated interferon lambda had lower viral loads by day 7 than those who received placebo. The incidence of adverse events was similar in the two groups.
CONCLUSIONS Among predominantly vaccinated outpatients with Covid-19, the incidence of hospitalization or an emergency department visit (observation for >6 hours) was significantly lower among those who received a single dose of pegylated interferon lambda than among those who received placebo. (Funded by FastGrants and others; TOGETHER ClinicalTrials.gov number, NCT04727424.
References
Andreakos, Salagianni, Galani, Koltsida, Interferon-λs: front-line guardians of immunity and homeostasis in the respiratory tract, Front Immunol
Banday, Stanifer, Florez-Vargas, Genetic regulation of OAS1 nonsense-mediated decay underlies association with COVID-19 hospitalization in patients of European and African ancestries, Nat Genet
Baños-Lara, Harvey, Mendoza, Impact and regulation of lambda interferon response in human metapneumovirus infection, J Virol
Chan, Ahn, Chang, Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: a randomized phase 2b study (LIRA-B), J Hepatol
Davidson, Mccabe, Crotta, IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment, EMBO Mol Med
Dinnon, Iii, Leist, Schäfer, A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures, Nature
Dodd, Freidlin, Korn, Platform trials -beware the noncomparable control group, N Engl J Med
Feld, Kandel, Biondi, Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial, Lancet Respir Med
Hakim, Chen, Ding, Basal interferon signaling and therapeutic use of interferons in controlling rotavirus infection in human intestinal cells and organoids, Sci Rep
Jagannathan, Andrews, Bonilla, Peginterferon lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial, Nat Commun
Montori, Permanyer-Miralda, Ferreira-González, Validity of composite end points in clinical trials, BMJ
Muir, Arora, Everson, A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection, J Hepatol
Nice, Baldridge, Mccune, Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity, Science
Orkin, Gill, Ghersi, Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement, JAMA
Prokunina-Olsson, Dickenson, COVID-19 and emerging viral infections: the case for interferon lambda, J Exp Med
Reis, Silva, Silva, A multicenter, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and highrisk for complications: the TOGETHER master trial protocol, Gates Open Research
Schandelmaier, Briel, Varadhan, Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses, CMAJ
Schulz, Altman, Moher, WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
DOI record:
{
"DOI": "10.1056/nejmoa2209760",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2209760",
"alternative-id": [
"10.1056/NEJMoa2209760"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4847-1034",
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"authenticated-orcid": false,
"family": "Reis",
"given": "Gilmar",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Moreira Silva",
"given": "Eduardo A.S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Medeiros Silva",
"given": "Daniela C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Thabane",
"given": "Lehana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Campos",
"given": "Vitoria H.S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Ferreira",
"given": "Thiago S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Santos",
"given": "Castilho V.Q.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Nogueira",
"given": "Ana M.R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Almeida",
"given": "Ana P.F.G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Savassi",
"given": "Leonardo C.M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Figueiredo-Neto",
"given": "Adhemar D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Dias",
"given": "Ana C.F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Freire Júnior",
"given": "Adelino M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Bitarães",
"given": "Carina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Milagres",
"given": "Aline C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Callegari",
"given": "Eduardo D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Simplicio",
"given": "Maria I.C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Ribeiro",
"given": "Luciene B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Oliveira",
"given": "Rosemary",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2901-0558",
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"authenticated-orcid": false,
"family": "Harari",
"given": "Ofir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Wilson",
"given": "Lindsay A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Forrest",
"given": "Jamie I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Ruton",
"given": "Hinda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Sprague",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "McKay",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Guo",
"given": "Christina M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Limbrick-Oldfield",
"given": "Eve H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Kanters",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Guyatt",
"given": "Gordon H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Rayner",
"given": "Craig R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Kandel",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Biondi",
"given": "Mia J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Kozak",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Hansen",
"given": "Bettina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Zahoor",
"given": "M. Atif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Arora",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Hislop",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Choong",
"given": "Ingrid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Feld",
"given": "Jordan J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Mills",
"given": "Edward J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From ViRx@Stanford, Stanford Biosecurity and Pandemic Preparedness Initiative (G.R., J.S.G., E.J.M.), and the Departments of Medicine (Division of Gastroenterology and Hepatology) and Microbiology and Immunology, Stanford University School of Medicine (J.S.G.), Stanford, and the Veterans Affairs Medical Center (J.S.G.) and Eiger BioPharmaceuticals (C.H., I.C.), Palo Alto — all in California; the Research Division, Cardresearch–Cardiologia Assistencial e de Pesquisa (G.R., E.A.S.M.S., D.C.M.S., V.H.S.C.,..."
}
],
"family": "Glenn",
"given": "Jeffrey S.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
2,
8
]
],
"date-time": "2023-02-08T22:01:02Z",
"timestamp": 1675893662000
},
"deposited": {
"date-parts": [
[
2023,
2,
8
]
],
"date-time": "2023-02-08T22:01:16Z",
"timestamp": 1675893676000
},
"funder": [
{
"DOI": "10.13039/100016608",
"award": [
"NA"
],
"doi-asserted-by": "publisher",
"name": "Rainwater Charitable Foundation"
},
{
"award": [
"NA"
],
"name": "Eiger BioPharmaceuticals"
},
{
"award": [
"NA"
],
"name": "FastGrants"
},
{
"DOI": "10.13039/100000865",
"award": [
"NA"
],
"doi-asserted-by": "publisher",
"name": "Bill and Melinda Gates Foundation"
},
{
"award": [
"NA"
],
"name": "FTX Foundation"
}
],
"indexed": {
"date-parts": [
[
2023,
2,
9
]
],
"date-time": "2023-02-09T05:38:32Z",
"timestamp": 1675921112051
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2023,
2,
9
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2023,
2,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
9
]
],
"date-time": "2023-02-09T00:00:00Z",
"timestamp": 1675900800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2209760",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "518-528",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2023,
2,
9
]
]
},
"published-print": {
"date-parts": [
[
2023,
2,
9
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.3389/fimmu.2017.01232",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1084/jem.20200653",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1016/j.jhep.2015.12.018",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1016/j.jhep.2014.07.022",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1128/JVI.02897-14",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1126/science.1258100",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.15252/emmm.201606413",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1038/s41598-018-26784-9",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1038/s41586-020-2708-8",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1016/S2213-2600(20)30566-X",
"doi-asserted-by": "publisher",
"key": "r10"
},
{
"DOI": "10.1038/s41467-021-22177-1",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.12688/gatesopenres.13304.2",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1136/bmj.c332",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1136/bmj.330.7491.594",
"doi-asserted-by": "publisher",
"key": "r15"
},
{
"DOI": "10.1001/jama.2021.9941",
"doi-asserted-by": "publisher",
"key": "r16"
},
{
"DOI": "10.1503/cmaj.200077",
"doi-asserted-by": "publisher",
"key": "r17"
},
{
"DOI": "10.1056/NEJMc2102446",
"doi-asserted-by": "publisher",
"key": "r18"
},
{
"DOI": "10.1038/s41588-022-01113-z",
"doi-asserted-by": "publisher",
"key": "r19"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2209760"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Early Treatment with Pegylated Interferon Lambda for Covid-19",
"type": "journal-article",
"volume": "388"
}