Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial
et al., The Lancet Global Health, doi:10.1016/S2214-109X(21)00448-4, TOGETHER, NCT04727424, Aug 2021 (preprint)
30th treatment shown to reduce risk in
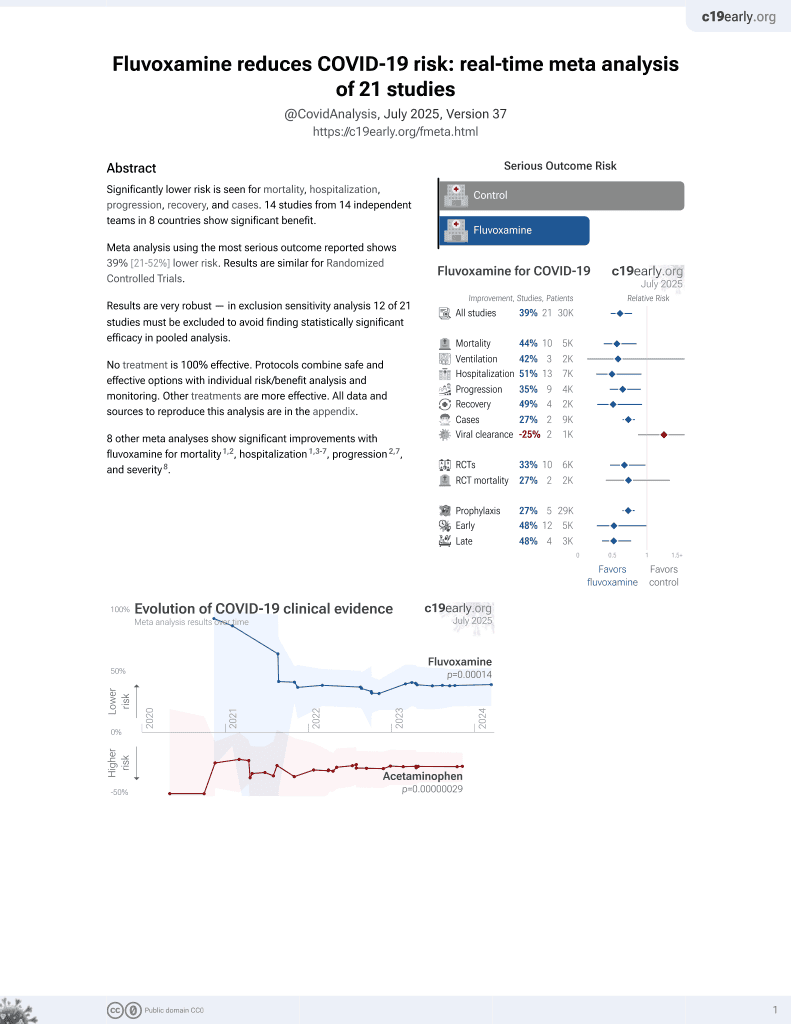
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
The TOGETHER trial has extreme COI, impossible data, blinding failure, randomization failure, uncorrected errors, and many protocol violations. Authors do not respond to these issues and they have refused to release the data as promised. Some issues may apply only to specific arms.
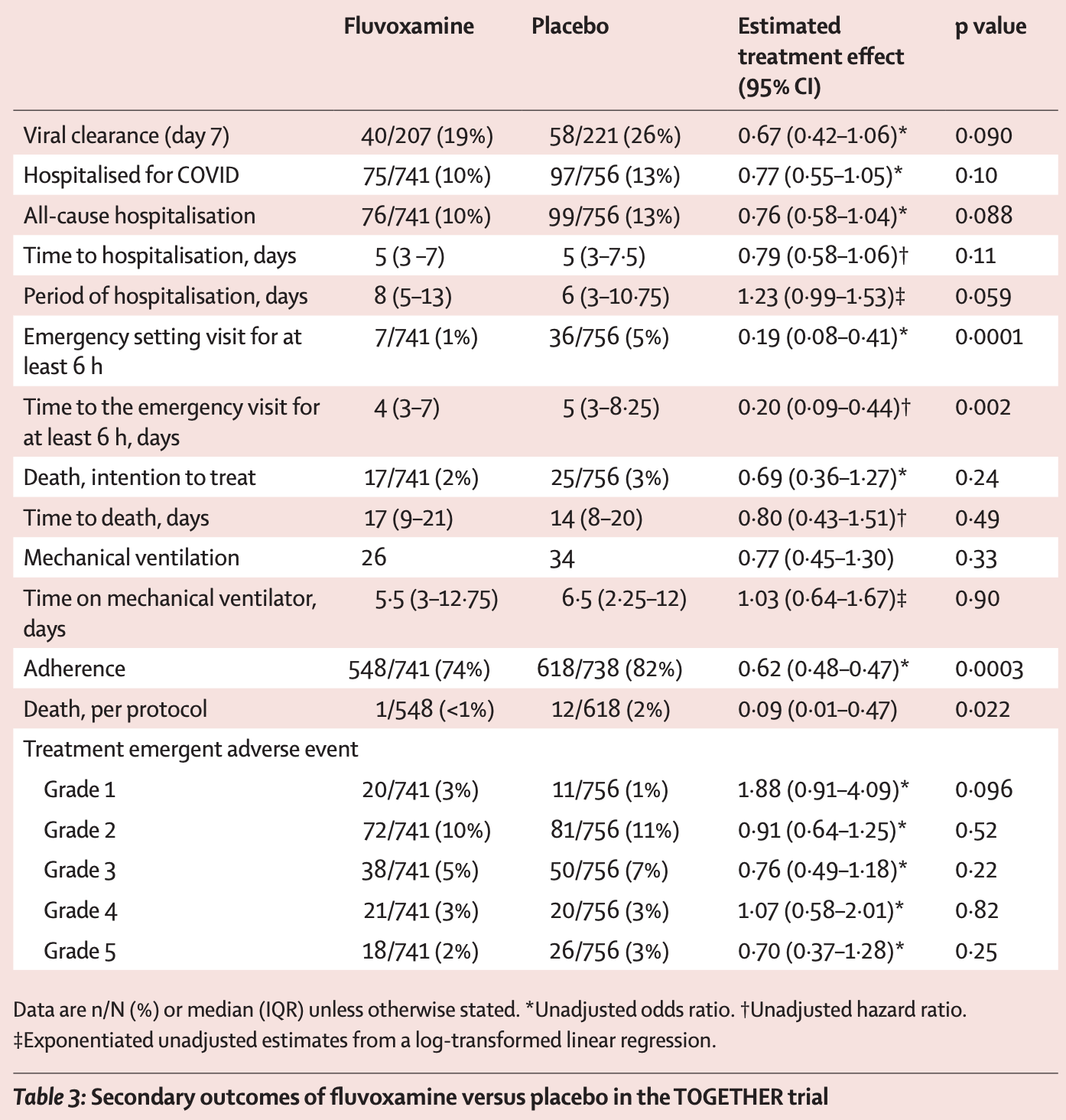
Together Trial showing significantly lower hospitalization/extended ER visits with fluvoxamine treatment. Adherence was only 73.2%. Symptom onset was unspecified or ≥4 days for 57% of patients. The schedule of study activities specifies treatment administration only one day after randomization, adding an additional day delay. Overall mortality is high for the patient population. Results may be impacted by late treatment, poor SOC, and may be specific to local variants1,2. Per-protocol analysis shows significantly improved results in this trial, however this may be subject to bias - the probability of adherence may be related to the probability of the outcome.
Regarding the combined hospitalization/extended ER observation outcome, authors have noted that at the study sites, extended medical observation was essentially equivalent to being hospitalized. “These were not standard emergency rooms but instead were COVID-19 emergency centers that were set up due to hospitals being overloaded,” Reiersen noted in an email to The Scientist. “A stay in these centers >6 hours was an indication that the patient was receiving care equivalent to hospitalization.”
Authors state "this study is only the second study to show an important treatment benefit for a repurposed drug in the early treatment population", however the actual number is at least 66 based on our database at the time of publication, using a conservative definition of at least 10% benefit (with statistical significance).
The total dose used is less than half of that in Lenze et al. There is an unusual amount of missing data - age is unknown for 6.5% of patients according to the sub-group analysis. Both age ≤50 and >50 show better results on the primary outcome than the overall result. The number of placebo patients changed significantly between the preprint and journal version. The number of treatment patients with viral clearance results reduced significantly between the preprint and journal version. Also see3.
Authors do not specify if the placebo looks identical to the film-coated Luvox tablets. Reportedly there is no registration of manufacturing for matching tablets by Abbott in Brazil, and no import license for identical placebo tablets abroad. This would be an additional reason for blinding failure if the placebo tablets are not identical in appearance.
The TOGETHER trial has extreme COI, impossible data,
blinding failure, randomization failure, uncorrected errors, and many
protocol violations. Authors do not respond to these issues and they
have refused to release the data as promised. Some issues may apply only
to specific arms. For more details see7-11.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
|
risk of death, 30.3% lower, RR 0.70, p = 0.24, treatment 17 of 741 (2.3%), control 25 of 756 (3.3%), NNT 99, odds ratio converted to relative risk, ITT.
|
|
risk of death, 90.8% lower, RR 0.09, p = 0.02, treatment 1 of 548 (0.2%), control 12 of 618 (1.9%), NNT 57, odds ratio converted to relative risk, per protocol.
|
|
risk of mechanical ventilation, 22.2% lower, RR 0.78, p = 0.33, treatment 26 of 741 (3.5%), control 34 of 756 (4.5%), NNT 101, odds ratio converted to relative risk, ITT.
|
|
risk of hospitalization, 21.6% lower, RR 0.78, p = 0.10, treatment 75 of 741 (10.1%), control 97 of 756 (12.8%), NNT 37, odds ratio converted to relative risk, ITT.
|
|
extended ER observation or hospitalization, 32.0% lower, RR 0.68, p = 0.004, treatment 79 of 741 (10.7%), control 119 of 756 (15.7%), NNT 20, ITT, primary outcome.
|
|
extended ER observation or hospitalization, 31.0% lower, RR 0.69, p = 0.006, treatment 78 of 740 (10.5%), control 115 of 752 (15.3%), NNT 21, mITT.
|
|
extended ER observation or hospitalization, 66.0% lower, RR 0.34, p < 0.001, treatment 541, control 609, per protocol.
|
|
risk of no viral clearance, 49.3% higher, RR 1.49, p = 0.09, treatment 167 of 207 (80.7%), control 163 of 221 (73.8%), adjusted per study, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
3.
web.archive.org, web.archive.org/web/*/https://twitter.com/Covid19Crusher/status/1430170252575395843.
4.
web.archive.org (B), web.archive.org/web/*/https://twitter.com/Covid19Crusher/status/1453726471499894787.
5.
web.archive.org (C), web.archive.org/web/*/https://twitter.com/Covid19Crusher/status/1453803654608269318.
7.
Reis et al., Effect of Early Treatment with Ivermectin among Patients with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2115869.
8.
Reis (B) et al., RETRACTED: Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: The TOGETHER randomized platform clinical trial, The Lancet Regional Health - Americas, doi:10.1016/j.lana.2021.100142.
9.
Reis (C) et al., Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19 The TOGETHER Randomized Clinical Trial, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.6468.
10.
Reis (D) et al., Early Treatment with Pegylated Interferon Lambda for Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2209760.
Reis et al., 23 Aug 2021, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 27 authors, study period 20 January, 2021 - 5 August, 2021, trial NCT04727424 (history) (TOGETHER).
Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial
The Lancet Global Health, doi:10.1016/s2214-109x(21)00448-4
Background Recent evidence indicates a potential therapeutic role of fluvoxamine for COVID-19. In the TOGETHER trial for acutely symptomatic patients with COVID-19, we aimed to assess the efficacy of fluvoxamine versus placebo in preventing hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to a tertiary hospital due to COVID-19. Methods This placebo-controlled, randomised, adaptive platform trial done among high-risk symptomatic Brazilian adults confirmed positive for SARS-CoV-2 included eligible patients from 11 clinical sites in Brazil with a known risk factor for progression to severe disease. Patients were randomly assigned (1:1) to either fluvoxamine (100 mg twice daily for 10 days) or placebo (or other treatment groups not reported here). The trial team, site staff, and patients were masked to treatment allocation. Our primary outcome was a composite endpoint of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 up to 28 days postrandom assignment on the basis of intention to treat. Modified intention to treat explored patients receiving at least 24 h of treatment before a primary outcome event and per-protocol analysis explored patients with a high level adherence (>80%). We used a Bayesian analytic framework to establish the effects along with probability of success of intervention compared with placebo. The trial is registered at ClinicalTrials.gov (NCT04727424) and is ongoing. Findings The study team screened 9803 potential participants for this trial. The trial was initiated on June 2, 2020, with the current protocol reporting randomisation to fluvoxamine from Jan 20 to Aug 5, 2021, when the trial arms were stopped for superiority. 741 patients were allocated to fluvoxamine and 756 to placebo. The average age of participants was 50 years (range 18-102 years); 58% were female. The proportion of patients observed in a COVID-19 emergency setting for more than 6 h or transferred to a teritary hospital due to COVID-19 was lower for the fluvoxamine group compared with placebo (79 [11%] of 741 vs 119 [16%] of 756); relative risk [RR] 0•68; 95% Bayesian credible interval [95% BCI]: 0•52-0•88), with a probability of superiority of 99•8% surpassing the prespecified superiority threshold of 97•6% (risk difference 5•0%). Of the composite primary outcome events, 87% were hospitalisations. Findings for the primary outcome were similar for the modified intention-to-treat analysis (RR 0•69, 95% BCI 0•53-0•90) and larger in the per-protocol analysis (RR 0•34, 95% BCI, 0•21-0•54). There were 17 deaths in the fluvoxamine group and 25 deaths in the placebo group in the primary intention-to-treat analysis (odds ratio [OR] 0•68, 95% CI: 0•36-1•27). There was one death in the fluvoxamine group and 12 in the placebo group for the perprotocol population (OR 0•09; 95% CI 0•01-0•47). We found no significant differences in number of treatment..
References
Anderson, Fluvoxamine, melatonin and COVID-19, Psychopharmacology (Berl)
Angus, Derde, Al-Beidh, Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial, JAMA
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Hoertel, Sánchez-Rico, Vernet, Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry, doi:10.1038/s41380-021-01021-4
Horby, Pessoa-Amorim, Peto, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial
Ishima, Fujita, Hashimoto, Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells, Eur J Pharmacol
Lenze, Mattar, Zorumski, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Omi, Tanimukai, Kanayama, Fluvoxamine alleviates ER stress via induction of sigma-1 receptor, Cell Death Dis
Pan, Peto, Henao-Restrepo, Repurposed antiviral drugs for Covid-19 -interim WHO Solidarity Trial results, N Engl J Med
Park, Siden, Zoratti, Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols, Trials
Pashaei, Drug repurposing of selective serotonin reuptake inhibitors: could these drugs help fight COVID-19 and save lives?, J Clin Neurosci
Rayner, Dron, Park, Accelerating clinical evaluation of repurposed combination therapies for COVID-19, Am J Trop Med Hyg
Reis, Silva Eadsm, Silva, A multi-center, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and high-risk for complications: the TOGETHER master trial protocol, Gates Open Res
Reis, Silva, Silva, Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial, JAMA Netw Open
Rosen, Seki, Fernández-Castañeda, Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis, Sci Transl Med
Schlienger, Meier, Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?, Am J Cardiovasc Drugs
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: a review of its mechanism of action and its role in COVID-19, doi:10.17605/OSF.IO/EG37X
Torres, Artaza, Profeta, Alonso, Kang, COVID-19 vaccination: returning to WHO's Health For All, Lancet Glob Health
Wang, Levi, Ellis, Hill, Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19, medRxiv, doi:10.1101/2021.06.01.21258147
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Who, World Health Organization model list of essential medicines: 21st list 2019
Woodcock, Lavange, Master protocols to study multiple therapies, multiple diseases, or both, N Engl J Med
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet
DOI record:
{
"DOI": "10.1016/s2214-109x(21)00448-4",
"ISSN": [
"2214-109X"
],
"URL": "http://dx.doi.org/10.1016/S2214-109X(21)00448-4",
"alternative-id": [
"S2214109X21004484"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Global Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2214-109X(21)00448-4"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2214-109X(21)00501-5"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Reis",
"given": "Gilmar",
"sequence": "first"
},
{
"affiliation": [],
"family": "dos Santos Moreira-Silva",
"given": "Eduardo Augusto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Daniela Carla Medeiros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thabane",
"given": "Lehana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milagres",
"given": "Aline Cruz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Thiago Santiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "dos Santos",
"given": "Castilho Vitor Quirino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Souza Campos",
"given": "Vitoria Helena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nogueira",
"given": "Ana Maria Ribeiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Almeida",
"given": "Ana Paula Figueiredo Guimaraes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Callegari",
"given": "Eduardo Diniz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Figueiredo Neto",
"given": "Adhemar Dias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Savassi",
"given": "Leonardo Cançado Monteiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simplicio",
"given": "Maria Izabel Campos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ribeiro",
"given": "Luciene Barra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliveira",
"given": "Rosemary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harari",
"given": "Ofir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forrest",
"given": "Jamie I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruton",
"given": "Hinda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sprague",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McKay",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Glushchenko",
"given": "Alla V",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rayner",
"given": "Craig R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lenze",
"given": "Eric J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reiersen",
"given": "Angela M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guyatt",
"given": "Gordon H",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mills",
"given": "Edward J",
"sequence": "additional"
}
],
"container-title": "The Lancet Global Health",
"container-title-short": "The Lancet Global Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
28
]
],
"date-time": "2021-10-28T06:18:26Z",
"timestamp": 1635401906000
},
"deposited": {
"date-parts": [
[
2022,
8,
9
]
],
"date-time": "2022-08-09T09:49:09Z",
"timestamp": 1660038549000
},
"indexed": {
"date-parts": [
[
2024,
4,
5
]
],
"date-time": "2024-04-05T08:29:04Z",
"timestamp": 1712305744841
},
"is-referenced-by-count": 272,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
24
]
],
"date-time": "2021-09-24T00:00:00Z",
"timestamp": 1632441600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2214109X21004484?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2214109X21004484?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e42-e51",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S2214-109X(20)30415-0",
"article-title": "COVID-19 vaccination: returning to WHO's Health For All",
"author": "Torres",
"doi-asserted-by": "crossref",
"first-page": "e1355",
"journal-title": "Lancet Glob Health",
"key": "10.1016/S2214-109X(21)00448-4_bib1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.20-0995",
"article-title": "Accelerating clinical evaluation of repurposed combination therapies for COVID-19",
"author": "Rayner",
"doi-asserted-by": "crossref",
"first-page": "1364",
"journal-title": "Am J Trop Med Hyg",
"key": "10.1016/S2214-109X(21)00448-4_bib2",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1038/cddis.2014.301",
"article-title": "Fluvoxamine alleviates ER stress via induction of sigma-1 receptor",
"author": "Omi",
"doi-asserted-by": "crossref",
"journal-title": "Cell Death Dis",
"key": "10.1016/S2214-109X(21)00448-4_bib3",
"volume": "5",
"year": "2014"
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: a review of its mechanism of action and its role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/S2214-109X(21)00448-4_bib4",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01021-4",
"article-title": "Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"journal-title": "Mol Psychiatry",
"key": "10.1016/S2214-109X(21)00448-4_bib5",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "10.1016/S2214-109X(21)00448-4_bib6",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s13063-019-3664-1",
"article-title": "Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "572",
"journal-title": "Trials",
"key": "10.1016/S2214-109X(21)00448-4_bib7",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1056/NEJMra1510062",
"article-title": "Master protocols to study multiple therapies, multiple diseases, or both",
"author": "Woodcock",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "N Engl J Med",
"key": "10.1016/S2214-109X(21)00448-4_bib8",
"volume": "377",
"year": "2017"
},
{
"DOI": "10.12688/gatesopenres.13304.2",
"article-title": "A multi-center, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and high-risk for complications: the TOGETHER master trial protocol",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Gates Open Res",
"key": "10.1016/S2214-109X(21)00448-4_bib9",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1007/s00213-020-05753-z",
"article-title": "Fluvoxamine, melatonin and COVID-19",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "611",
"journal-title": "Psychopharmacology (Berl)",
"key": "10.1016/S2214-109X(21)00448-4_bib10",
"volume": "238",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity Trial results",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "10.1016/S2214-109X(21)00448-4_bib11",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.6468",
"article-title": "Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S2214-109X(21)00448-4_bib12",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Lancet",
"key": "10.1016/S2214-109X(21)00448-4_bib13",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1016/j.jocn.2021.03.010",
"article-title": "Drug repurposing of selective serotonin reuptake inhibitors: could these drugs help fight COVID-19 and save lives?",
"author": "Pashaei",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "J Clin Neurosci",
"key": "10.1016/S2214-109X(21)00448-4_bib14",
"volume": "88",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2014.01.064",
"article-title": "Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells",
"author": "Ishima",
"doi-asserted-by": "crossref",
"first-page": "167",
"journal-title": "Eur J Pharmacol",
"key": "10.1016/S2214-109X(21)00448-4_bib15",
"volume": "727",
"year": "2014"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"article-title": "Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis",
"author": "Rosen",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S2214-109X(21)00448-4_bib16",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.2165/00129784-200303030-00001",
"article-title": "Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?",
"author": "Schlienger",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Am J Cardiovasc Drugs",
"key": "10.1016/S2214-109X(21)00448-4_bib17",
"volume": "3",
"year": "2003"
},
{
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial",
"author": "Horby",
"journal-title": "medRxiv",
"key": "10.1016/S2214-109X(21)00448-4_bib18",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial",
"author": "Angus",
"doi-asserted-by": "crossref",
"first-page": "1317",
"journal-title": "JAMA",
"key": "10.1016/S2214-109X(21)00448-4_bib19",
"volume": "324",
"year": "2020"
},
{
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"journal-title": "BMJ",
"key": "10.1016/S2214-109X(21)00448-4_bib20",
"volume": "370",
"year": "2020"
},
{
"article-title": "Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19",
"author": "Wang",
"journal-title": "medRxiv",
"key": "10.1016/S2214-109X(21)00448-4_bib21",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "10.1016/S2214-109X(21)00448-4_bib22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S2214-109X(21)00448-4_bib23",
"volume": "384",
"year": "2021"
},
{
"key": "10.1016/S2214-109X(21)00448-4_bib24",
"series-title": "World Health Organization model list of essential medicines: 21st list 2019",
"year": "2019"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2214109X21004484"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}