Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV)
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiad275, PLATCOV, NCT05041907, Jul 2023
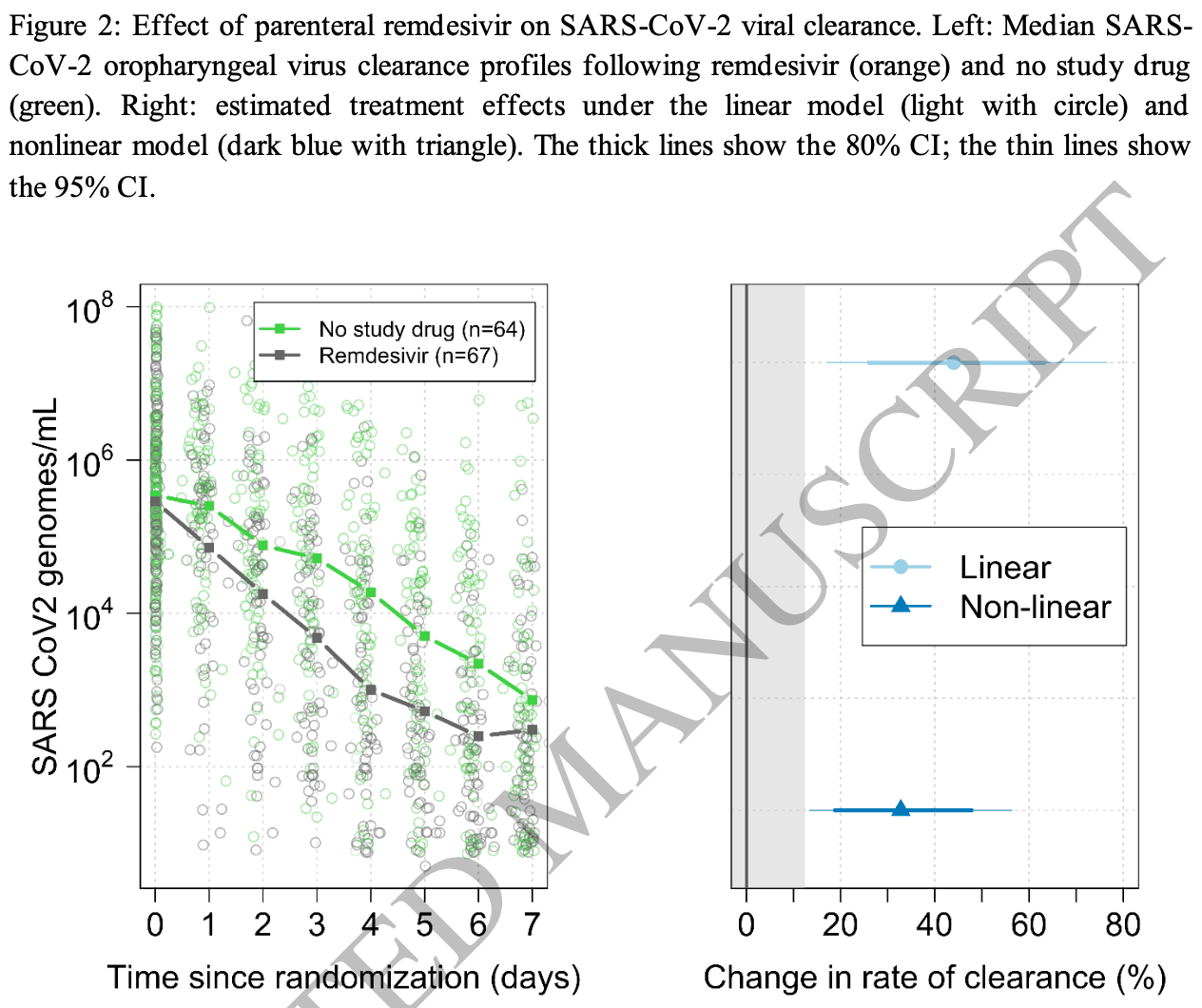
High conflict of interest RCT with very low risk patients with high existing immunity, showing faster viral clearance with remdesivir. The viral clearance half-life was very short in both arms. With rapid viral clearance and very low risk patients, the trial favors detecting an effect with intravenous treatments that have rapid onset of action.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
|
risk of hospitalization, 66.3% lower, RR 0.34, p = 1.00, treatment 0 of 67 (0.0%), control 1 of 69 (1.4%), NNT 69, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
relative clearance half-life, 28.9% better, RR 0.71, p < 0.001, treatment median 12.8 IQR 8.0 n=67, control median 18.0 IQR 10.5 n=69, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Jittamala et al., 20 Jul 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 30.1, 42 authors, study period 30 September, 2021 - 10 June, 2022, average treatment delay 2.4 days, trial NCT05041907 (history) (PLATCOV).
Contact: william@tropmedres.ac, nickw@tropmedres.ac.
Clinical Antiviral Efficacy of Remdesivir in Coronavirus Disease 2019: An Open-Label, Randomized Controlled Adaptive Platform Trial (PLATCOV)
The Journal of Infectious Diseases, doi:10.1093/infdis/jiad275
Background: Uncertainty over the therapeutic benefit provided by parenteral remdesivir in COVID-19 has resulted in varying treatment guidelines.
Methods: In a multicenter open label, controlled, adaptive, pharmacometric platform trial, lowrisk adult patients with early symptomatic COVID-19 were randomized to one of eight treatment arms including intravenous remdesivir (200mg followed by 100mg daily for five days) or no study drug. The primary outcome was the rate of viral clearance (estimated under a linear model fit to the daily log10 viral densities, days 0-7) in standardized duplicate oropharyngeal swab eluates, in a modified intention-to-treat population (mITT). This ongoing adaptive trial is registered at ClinicalTrials.gov (NCT05041907).
Results: The two study arms enrolled 131 patients (remdesivir n=67, no study drug n=64) and estimated viral clearance rates from a median of 18 swab samples per patient (a total of 2356 qPCRs). Under the linear model, compared with the contemporaneous control arm (no study drug), remdesivir accelerated mean estimated SARS-CoV-2 viral clearance by 42% (95% credible interval [CI] 18 to 73). Interpretation: Parenteral remdesivir accelerates viral clearance in early symptomatic COVID-19. Pharmacometric assessment of therapeutics using the described method can rapidly and efficiently determine in vivo clinical efficacy.
References
Abd-Elsalam, Salama, Soliman, Naguib, Ibrahim et al., Remdesivir Efficacy in COVID-19 Treatment: A Randomized Controlled Trial, Am J Trop Med Hyg
Ader, Bouscambert-Duchamp, Hites, Peiffer-Smadja, Poissy et al., Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, Lancet Infect Dis
Ali, Azher, Baqi, Borgia, Carrier et al., None, CMAJ
Beigel, Tomashek, Dodd, Mehta, Zingman et al., ACTT-1Study Group Members. Remdesivir for the Treatment of Covid-19 -Final Report, NEngl J Med
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., MOVe-OUT Study Group. Molnupiravir for Oral Treatment of Covid -19 in Nonhospitalized Patients, N Engl J Med
Fischer Wa 2nd, Eron, Jr, Holman, Cohen et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Goldman, Lye, Hui, Marks, Bruno et al., GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., EPIC-HR Investigators. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Lee, Murthy, Corpo, Senécal, Butler-Laporte et al., Remdesivir for the treatment of COVID-19: a systematic review and metaanalysis, Clin Microbiol Infect
Paules, Fauci, COVID-19: The therapeutic landscape, Med (N Y)
Schilling, Jittamala, Watson, Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, doi:10.7554/eLife.83201
Solera, Árbol, Bahinskaya, Marks, Humar et al., Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave, Am J Transplant, doi:10.1111/ajt.17199
Spinner, Gottlieb, Criner, López, Cattelan et al., GS-US-540-5774 Investigators. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial, JAMA
Wang, Zhang, Du, Du, Zhao et al., None, Lancet
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 Viral Clearance Kinetics to Improve the Design of Antiviral Pharmacometric Studies, Antimicrob Agents Chemother, doi:10.1128/aac.00192-22
Weinreich, Sivapalasingam, Norton, Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge: A Cohort Study and Randomized Comparative Effectiveness Trial, JAMA Netw Open
White, Strub-Wourgaft, Faiz, Guerin, Guidelines should not pool evidence from uncomplicated and severe COVID-19, Lancet
Who Solidarity, Consortium, Pan, Peto, Henao-Restrepo et al., None, N Engl J Med
Williamson, Feldmann, Schwarz, Meade-White, Porter et al., de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2
DOI record:
{
"DOI": "10.1093/infdis/jiad275",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiad275",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Uncertainty over the therapeutic benefit of parenteral remdesivir in coronavirus disease 2019 (COVID-19) has resulted in varying treatment guidelines.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In a multicenter open-label, controlled, adaptive, pharmacometric platform trial, low-risk adult patients with early symptomatic COVID-19 were randomized to 1 of 8 treatment arms including intravenous remdesivir (200 mg followed by 100 mg daily for 5 days) or no study drug. The primary outcome was the rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) clearance (estimated under a linear model fit to the daily log10 viral densities, days 0–7) in standardized duplicate oropharyngeal swab eluates, in a modified intention-to-treat population. This ongoing adaptive trial is registered at ClinicalTrials.gov (NCT05041907).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The 2 study arms enrolled 131 patients (remdesivir n = 67, no study drug n = 64) and estimated viral clearance rates from a median of 18 swab samples per patient (a total of 2356 quantitative polymerase chain reactions). Under the linear model, compared with the contemporaneous control arm (no study drug), remdesivir accelerated mean estimated viral clearance by 42% (95% credible interval, 18%–73%).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Parenteral remdesivir accelerates viral clearance in early symptomatic COVID-19. Pharmacometric assessment of therapeutics using the method described can determine in vivo clinical antiviral efficacy rapidly and efficiently.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Jittamala",
"given": "Podjanee",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6328-8748",
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"authenticated-orcid": false,
"family": "Schilling",
"given": "William H K",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5524-0325",
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"authenticated-orcid": false,
"family": "Watson",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Luvira",
"given": "Viravarn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Siripoon",
"given": "Tanaya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Ngamprasertchai",
"given": "Thundon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit, Centre for Advanced and Innovative Therapies , Belo Horizonte , Brazil"
}
],
"family": "Almeida",
"given": "Pedro J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Ekkapongpisit",
"given": "Maneerat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Cruz",
"given": "Cintia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Callery",
"given": "James J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Boyd",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Anunsittichai",
"given": "Orawan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Hongsuwan",
"given": "Maliwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Singhaboot",
"given": "Yutatirat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Pagornrat",
"given": "Watcharee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Tuntipaiboontana",
"given": "Runch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Kruabkontho",
"given": "Varaporn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Ngernseng",
"given": "Thatsanun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Tubprasert",
"given": "Jaruwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Abdad",
"given": "Mohammad Yazid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Keayarsa",
"given": "Srisuda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
}
],
"family": "Madmanee",
"given": "Wanassanan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Genetics, Ecology and Evolution, Institute of Biological Sciences, Universidade Federal de Minas Gerais , Belo Horizonte , Brazil"
}
],
"family": "Aguiar",
"given": "Renato S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Genetics, Ecology and Evolution, Institute of Biological Sciences, Universidade Federal de Minas Gerais , Belo Horizonte , Brazil"
}
],
"family": "Santos",
"given": "Franciele M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Batty",
"given": "Elizabeth M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bangplee Hospital, Ministry of Public Health , Samut Prakarn , Thailand"
}
],
"family": "Hanboonkunupakarn",
"given": "Pongtorn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Hanboonkunupakarn",
"given": "Borimas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bangplee Hospital, Ministry of Public Health , Samut Prakarn , Thailand"
}
],
"family": "Sookprome",
"given": "Sakol",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Poovorawan",
"given": "Kittiyod",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Molecular Tropical Medicine and Genetics, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Imwong",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Taylor",
"given": "Walter R J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Navamindradhiraj University , Bangkok , Thailand"
}
],
"family": "Chotivanich",
"given": "Vasin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Science and Health Technology, Navamindradhiraj University , Bangkok , Thailand"
}
],
"family": "Sangketchon",
"given": "Chunlanee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Navamindradhiraj University , Bangkok , Thailand"
}
],
"family": "Ruksakul",
"given": "Wiroj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Chotivanich",
"given": "Kesinee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Pukrittayakamee",
"given": "Sasithon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5190-2395",
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"authenticated-orcid": false,
"family": "Dondorp",
"given": "Arjen M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "Day",
"given": "Nicholas P J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit, Centre for Advanced and Innovative Therapies , Belo Horizonte , Brazil"
}
],
"family": "Teixeira",
"given": "Mauro M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2905-1034",
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"authenticated-orcid": false,
"family": "Piyaphanee",
"given": "Watcharapong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University , Bangkok , Thailand"
}
],
"family": "Phumratanaprapin",
"given": "Weerapong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mahidol Oxford Tropical Medicine Research Unit , Bangkok , Thailand"
},
{
"name": "Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford , Oxford , United Kingdom"
}
],
"family": "White",
"given": "Nicholas J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "White",
"given": "Nicholas J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schilling",
"given": "William H K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luvira",
"given": "Viravarn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Callery",
"given": "James J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Day",
"given": "Nicholas P J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pukrittayakamee",
"given": "Sasithon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyd",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruz",
"given": "Cintia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dondorp",
"given": "Arjen M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taylor",
"given": "Walter R J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watson",
"given": "James A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piyaphanee",
"given": "Watcharapong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poovorawan",
"given": "Kittiyod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngamprasertchai",
"given": "Thundon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siripoon",
"given": "Tanaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Borimas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Kesinee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jittamala",
"given": "Podjanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imwong",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ekkapongpisit",
"given": "Maneerat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruabkontho",
"given": "Varaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngernseng",
"given": "Thatsanun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tubprasert",
"given": "Jaruwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdad",
"given": "Mohammad Yazid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keayarsa",
"given": "Srisuda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anunsittichai",
"given": "Orawan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hongsuwan",
"given": "Maliwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singhaboot",
"given": "Yutatirat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madmanee",
"given": "Wanassanan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Batty",
"given": "Elizabeth M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuntipaiboontana",
"given": "Runch",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pagornrat",
"given": "Watcharee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Vasin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruksakul",
"given": "Wiroj",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sangketchon",
"given": "Chunlanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Pongtorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sookprome",
"given": "Sakol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Mauro M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almeida",
"given": "Pedro J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguiar",
"given": "Renato S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Franciele M",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the PLATCOV Collaborative Group",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
19
]
],
"date-time": "2023-07-19T23:29:15Z",
"timestamp": 1689809355000
},
"deposited": {
"date-parts": [
[
2023,
11,
12
]
],
"date-time": "2023-11-12T12:17:50Z",
"timestamp": 1699791470000
},
"funder": [
{
"DOI": "10.13039/100010269",
"award": [
"223195/Z/21/Z)"
],
"doi-asserted-by": "publisher",
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
8
]
],
"date-time": "2024-03-08T09:19:10Z",
"timestamp": 1709889550424
},
"is-referenced-by-count": 3,
"issue": "10",
"issued": {
"date-parts": [
[
2023,
7,
20
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2023,
7,
20
]
]
},
"published-print": {
"date-parts": [
[
2023,
11,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
20
]
],
"date-time": "2023-07-20T00:00:00Z",
"timestamp": 1689811200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiad275/51029858/jiad275.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/228/10/1318/53307833/jiad275.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/228/10/1318/53307833/jiad275.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "1318-1325",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
7,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
20
]
]
},
"published-other": {
"date-parts": [
[
2023,
11,
15
]
]
},
"published-print": {
"date-parts": [
[
2023,
11,
11
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "2023111212172889600_jiad275-B1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial",
"author": "Spinner",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "JAMA",
"key": "2023111212172889600_jiad275-B3",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results",
"author": "WHO Solidarity Trial Consortium",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B4",
"volume": "384",
"year": "2021"
},
{
"author": "World Health Organization",
"key": "2023111212172889600_jiad275-B5",
"year": "2022"
},
{
"author": "World Health Organization",
"key": "2023111212172889600_jiad275-B6",
"year": "2023"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses",
"author": "WHO Solidarity Trial Consortium",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "Lancet",
"key": "2023111212172889600_jiad275-B7",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00469-4",
"article-title": "Guidelines should not pool evidence from uncomplicated and severe COVID-19",
"author": "White",
"doi-asserted-by": "crossref",
"first-page": "1262",
"journal-title": "Lancet",
"key": "2023111212172889600_jiad275-B8",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.medj.2021.04.015",
"article-title": "COVID-19: the therapeutic landscape",
"author": "Paules",
"doi-asserted-by": "crossref",
"first-page": "493",
"journal-title": "Med",
"key": "2023111212172889600_jiad275-B9",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.04.018",
"article-title": "Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1203",
"journal-title": "Clin Microbiol Infect",
"key": "2023111212172889600_jiad275-B11",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"article-title": "A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus",
"author": "Fischer",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "2023111212172889600_jiad275-B12",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B13",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B14",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17199",
"article-title": "Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the Omicron BA.2 wave",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "78",
"journal-title": "Am J Transplant",
"key": "2023111212172889600_jiad275-B15",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1128/aac.00192-22",
"article-title": "Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies",
"author": "Watson",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "2023111212172889600_jiad275-B16",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.7554/eLife.83201",
"article-title": "Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV)",
"author": "Schilling",
"doi-asserted-by": "crossref",
"journal-title": "ELife",
"key": "2023111212172889600_jiad275-B17",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 days in patients with severe Covid-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B18",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(21)00485-0",
"article-title": "Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial",
"author": "Ader",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Lancet Infect Dis",
"key": "2023111212172889600_jiad275-B19",
"volume": "22",
"year": "2022"
},
{
"article-title": "Remdesivir efficacy in COVID-19 treatment: a randomized controlled trial",
"author": "Abd-Elsalam",
"first-page": "886",
"journal-title": "Am J Trop Med Hyg",
"key": "2023111212172889600_jiad275-B20",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1503/cmaj.211698",
"article-title": "Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "E242",
"journal-title": "CMAJ",
"key": "2023111212172889600_jiad275-B21",
"volume": "194",
"year": "2022"
},
{
"author": "Gilead Sciences, Inc",
"key": "2023111212172889600_jiad275-B22",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2423-5",
"article-title": "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Nature",
"key": "2023111212172889600_jiad275-B23",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "2023111212172889600_jiad275-B24",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.20957",
"article-title": "Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge: a cohort study and randomized comparative effectiveness trial",
"author": "Huang",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "2023111212172889600_jiad275-B25",
"volume": "5",
"year": "2022"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/228/10/1318/7226765"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Clinical Antiviral Efficacy of Remdesivir in Coronavirus Disease 2019: An Open-Label, Randomized Controlled Adaptive Platform Trial (PLATCOV)",
"type": "journal-article",
"volume": "228"
}