Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial
et al., BMC Infectious Diseases, doi:10.1186/s12879-023-08835-3, PLATCOV, NCT05041907, Apr 2023 (preprint)
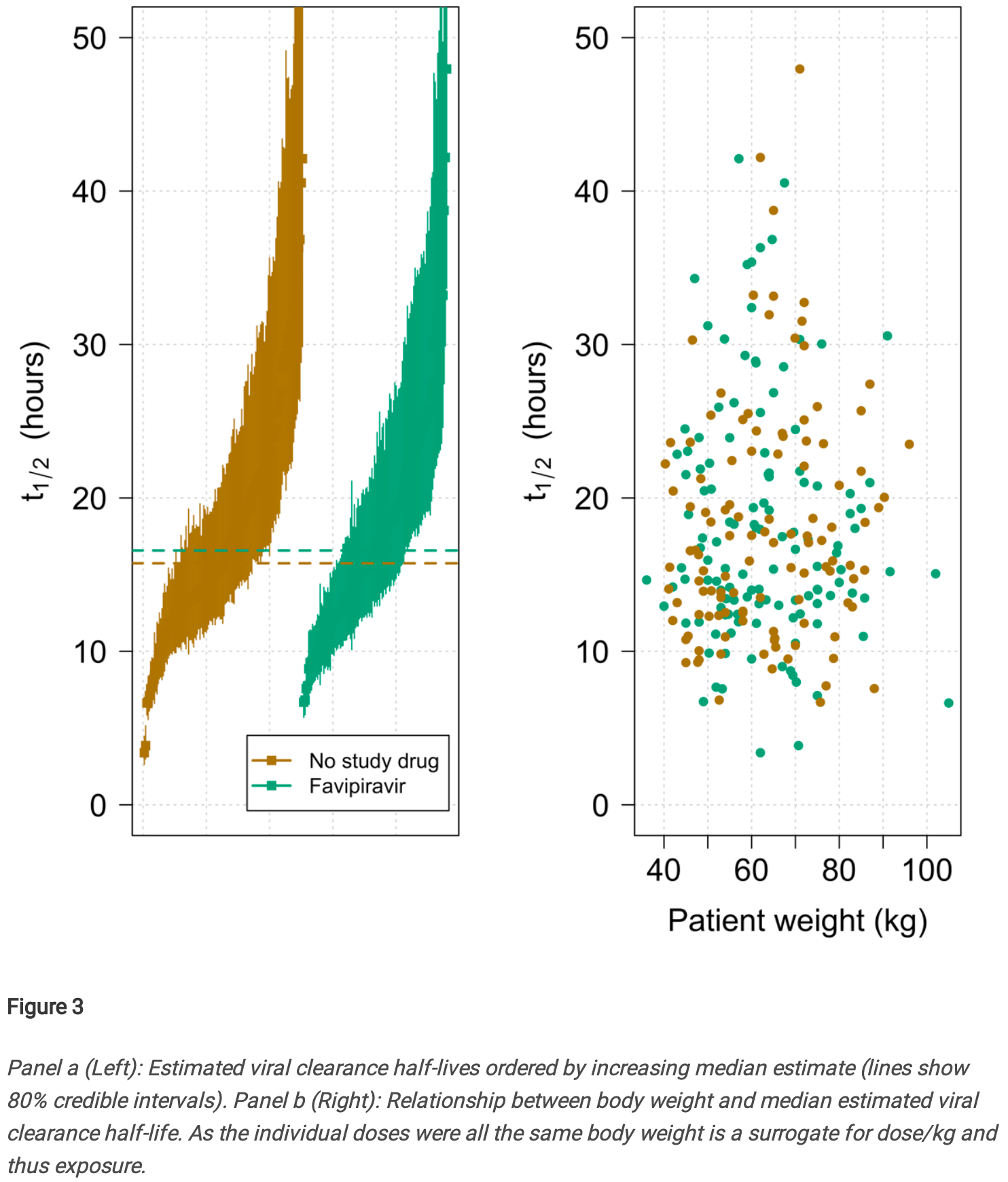
High conflict of interest RCT with very low risk patients, high existing immunity, and a post-hoc change to exclude patients more likely to benefit. There was no significant difference in viral clearance with favipiravir among patients with high viral load at baseline. Patients in both arms had very short viral clearance half-life times.
With rapid viral clearance and very low risk patients, infection is less likely to spread to other tissues. Systemic treatment is less applicable, and has less time to reach therapeutic concentrations before self-recovery.
Treatment administered directly to the respiratory tract, e.g. as in1, may be more effective for COVID-19 in general, and extend applicability to fast-resolving cases with infection primarily localized to the respiratory tract.
Authors note that "all-cause hospitalisation for clinical deterioration (until day 28) was a secondary endpoint", but do not provide the result.
For more discussion of the post-hoc change and other issues see2.
Potential risks of favipiravir include kidney injury3-5, liver injury4-7, cardiovascular events7,8, pulmonary toxicity8,9, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants10-16.
|
relative clearance rate, 5.7% worse, RR 1.06, p = 0.42, treatment median 16.6 IQR 10.0 n=116, control median 15.7 IQR 13.0 n=132, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Yildiz Pekoz et al., Pulmonary delivery of favipiravir inhalation solution for COVID-19 treatment: in vitro characterization, stability, in vitro cytotoxicity, and antiviral activity using real time cell analysis, Drug Delivery, doi:10.1080/10717544.2022.2118398.
2.
Schilling et al., Pharmacometrics of high dose ivermectin in early COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV), eLife, doi:10.7554/eLife.83201.
3.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
4.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
5.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
6.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
7.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
8.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
9.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
10.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
11.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
12.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
13.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
14.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Luvira et al., 5 Apr 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 30.1, 36 authors, study period 30 September, 2021 - 31 October, 2022, trial NCT05041907 (history) (PLATCOV).
Contact: william@tropmedres.ac, nickw@tropmedres.ac.
Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial
BMC Infectious Diseases, doi:10.1186/s12879-023-08835-3
Brief summary In early symptomatic COVID-19 treatment, high dose oral favipiravir did not accelerate viral clearance. Background Favipiravir, an anti-influenza drug, has in vitro antiviral activity against SARS-CoV-2. Clinical trial evidence to date is inconclusive. Favipiravir has been recommended for the treatment of COVID-19 in some countries.
Methods In a multicentre open-label, randomised, controlled, adaptive platform trial, low-risk adult patients with early symptomatic COVID-19 were randomised to one of ten treatment arms including high dose oral favipiravir (3.6g on day 0 followed by 1.6g daily to complete 7 days treatment) or no study drug. The primary outcome was the rate of viral clearance (derived under a linear mixed-effects model from the daily log 10 viral densities in standardised duplicate oropharyngeal swab eluates taken daily over 8 days [18 swabs per patient]), assessed in a modified intention-to-treat population (mITT). The safety population included all patients who received at least one dose of the allocated intervention. This ongoing adaptive platform trial was registered at ClinicalTrials.gov (NCT05041907) on 13/09/2021. Results In the final analysis, the mITT population contained data from 114 patients randomised to favipiravir and 126 patients randomised concurrently to no study drug. Under the linear mixed-effects model fitted to all oropharyngeal viral density estimates in the first 8 days from randomisation (4,318 swabs), there was no difference in the rate of viral †
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-023-08835-3. Authors' contributions V.L., J.A.W., S.B., W.H.K.S., and N.J.W wrote the first draft of the manuscript. P.J., V.L., T.S., T.N., B.H., S.S., K.P., P.B., V.C., P.J.A., M.M., S.P., W.P., W.P. were responsible for collection of clinical data. T.N. was responsible for data curation. J.A.W. was responsible for statistical analysis and the figures. S.K., W.M., M.Y.A., R.A.S., F.M.S., R.T., M.I., K.C. were responsible for laboratory testing and analysis. V.K., J.T., were responsible for trial set-up and monitoring. C.C. was responsible for coordination of the study in Brazil and J.J.C. and S.B. for safety monitoring and document preparation. W.R.J.T., A.M.D, N.P.J.D, N.J.W supervised the study and gave scientific input. All authors reviewed the manuscript.
Additional file 1: Supplementary
Availability of data and materials All code and data are openly accessible via GitHub: https:// github. com/ jwato watson/ PLATC OV-Favip iravir. The final datasets will be stored locally and securely at the Mahidol Oxford Research Unit for long-term storage and access. Additional anonymised participant data can be made available by request on a case-by-case basis from the MORU Data Access Committee at datasharing@tropmedres.ac and can be made available by request to the corresponding author.
Declarations Ethics approval and consent to participate
..
References
Abdulrahman, Odat, Tayar, Rana, Alharthy, Favipiravir efficacy and safety for the treatment of severe coronavirus disease 2019: a retrospective study, J Ayub Med Coll Abbottabad
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Bosaeed, Alharbi, Alrehily, Bahlaq, Gaifer, Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial, Clin Microbiol Infect
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of early treatment with favipiravir on disease progression among high-risk patients with coronavirus disease 2019 (COVID-19): a randomized openlabel clinical trial, Clin Infect Dis
Coomes, Haghbayan, Favipiravir, an antiviral for COVID-19?, J Antimicrob Chemother
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., Prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob Agents Chemother
Doi, Ishihara, Banno, Ando, Kondo, Favipiravir Observational Study. Favipiravir for symptomatic COVID-19: a nationwide observational cohort study, J Infect Chemother
Driouich, Cochin, Lingas, Moureau, Touret et al., Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun
Du, Chen, Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin Pharmacol Ther
Fischer Wa 2nd, Eron, Jr, Holman, Cohen et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res
Furuta, Takahashi, Fukuda, Kuno, Kamiyama et al., In vitro and in vivo activities of anti-influenza virus compound T-705, Antimicrob Agents Chemother
Golan, Campos, Woolson, Cilla, Hanabergh et al., Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial, Clin Infect Dis
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med
Hassaniazad, Farshidi, Gharibzadeh, Bazram, Khalili et al., Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: a randomized clinical trial, J Med Virol
Holubar, Subramanian, Purington, Hedlin, Bunning et al., Favipiravir for treatment of outpatients with asymptomatic or uncomplicated coronavirus disease 2019: a double-blind, randomized, placebo-controlled, phase 2 trial, Clin Infect Dis
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase ii/iii multicenter randomized clinical trial, Clin Infect Dis
Jittamala, Schilling, Watson, Luvira, Siripoon et al., Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV), J Infect Dis, doi:10.1093/infdis/jiad275
Kaptein, Jacobs, Langendries, Seldeslachts, Horst et al., Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci
Khamis, Naabi, Lawati, Ambusaidi, Sharji et al., Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia, Int J Infect Dis
Lowe, Brown, Chowdhury, Davey, Yee et al., Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): a randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19, PLoS Med
Mcmahon, Lau, Coldham, Roney, Hagenauer et al., Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial, EClinicalMedicine
Nguyen, Guedj, Anglaret, Laouénan, Madelain et al., Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted, PLoS Negl Trop Dis
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Covid-19 phase 3 prevention trial team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Qahtani, Kumar, Aljawder, Abdulrahman, Mohamed et al., Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease, Sci Rep
Reddy, Patil, Khobragade, Balki, Raj et al., Evaluation of the safety and efficacy of favipiravir in adult Indian patients with mild-to-moderate COVID-19 in a real-world setting, Int J Gen Med
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with Covid-19, N Engl J Med
Schilling, Jittamala, Watson, Boyd, Luvira et al., Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an openlabel, phase 2, randomised, controlled, adaptive trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00493-0
Schilling, Jittamala, Watson, Ekkapongpisit, Siripoon et al., Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV), Elife, doi:10.7554/eLife.83201
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study, Emerg Microbes Infect
Sissoko, Laouenan, Folkesson, Lebing, Beavogui et al., Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med
Sitasuwan, Phisalprapa, Srivanichakorn, Washirasaksiri, Auesomwang et al., Early antiviral and supervisory dexamethasone treatment improve clinical outcomes of nonsevere COVID-19 patients, Medicine
Sleeman, Mishin, Deyde, Furuta, Klimov et al., In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses, Antimicrob Agents Chemother
Solaymani-Dodaran, Ghanei, Bagheri, Qazvini, Vahedi et al., Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia, Int Immunopharmacol
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Wu, Wang, Gao, Liu et al., Structural basis for RNA replication by the SARS-CoV-2 polymerase, Cell
Waters, Warren, Hughes, Lewis, Zhang, Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environ Mol Mutagen
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies, Antimicrob Agents Chemother
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Zhao, Zhang, Zhu, Chen, Chen et al., Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial, Int Immunopharmacol
DOI record:
{
"DOI": "10.1186/s12879-023-08835-3",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-023-08835-3",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Brief summary</jats:title>\n <jats:p>In early symptomatic COVID-19 treatment, high dose oral favipiravir did not accelerate viral clearance.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Favipiravir, an anti-influenza drug, has in vitro antiviral activity against SARS-CoV-2. Clinical trial evidence to date is inconclusive. Favipiravir has been recommended for the treatment of COVID-19 in some countries.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In a multicentre open-label, randomised, controlled, adaptive platform trial, low-risk adult patients with early symptomatic COVID-19 were randomised to one of ten treatment arms including high dose oral favipiravir (3.6g on day 0 followed by 1.6g daily to complete 7 days treatment) or no study drug. The primary outcome was the rate of viral clearance (derived under a linear mixed-effects model from the daily log<jats:sub>10</jats:sub> viral densities in standardised duplicate oropharyngeal swab eluates taken daily over 8 days [18 swabs per patient]), assessed in a modified intention-to-treat population (mITT). The safety population included all patients who received at least one dose of the allocated intervention. This ongoing adaptive platform trial was registered at ClinicalTrials.gov (NCT05041907) on 13/09/2021.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In the final analysis, the mITT population contained data from 114 patients randomised to favipiravir and 126 patients randomised concurrently to no study drug. Under the linear mixed-effects model fitted to all oropharyngeal viral density estimates in the first 8 days from randomisation (4,318 swabs), there was no difference in the rate of viral clearance between patients given favipiravir and patients receiving no study drug; a -1% (95% credible interval: -14 to 14%) difference. High dose favipiravir was well-tolerated.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Interpretation</jats:title>\n <jats:p>Favipiravir does not accelerate viral clearance in early symptomatic COVID-19. The viral clearance rate estimated from quantitative measurements of oropharyngeal eluate viral densities assesses the antiviral efficacy of drugs in vivo with comparatively few studied patients.</jats:p>\n </jats:sec>",
"alternative-id": [
"8835"
],
"article-number": "89",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "10 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "21 November 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "15 January 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "All patients provided fully informed written consent. The trial was approved by local and national research ethics boards in Thailand (Faculty of Tropical Medicine Ethics Committee, Mahidol University, FTMEC Ref: TMEC 21–058) and the Central Research Ethics Committee (CREC, Bangkok, Thailand, CREC Ref: CREC048/64BP-MED34), in Brazil by the Research Ethics Committee of the Universidade Federal de Minas Gerais (COEP-UFMG, Minas Gerais, Brazil, COEP-UFMG) and National Research Ethics Commission- (CONEP, Brazil, COEP-UFMG and CONEP Ref: CAAE:51593421.1.0000.5149), and by the Oxford University Tropical Research Ethics Committee (OxTREC, Oxford, UK, OxTREC Ref: 24–21). All methods were performed in accordance with the relevant guidelines and regulations (e.g. Declaration of Helsinki)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Luvira",
"given": "Viravarn",
"sequence": "first"
},
{
"affiliation": [],
"family": "Schilling",
"given": "William H. K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jittamala",
"given": "Podjanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watson",
"given": "James A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyd",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siripoon",
"given": "Tanaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngamprasertchai",
"given": "Thundon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almeida",
"given": "Pedro J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ekkapongpisit",
"given": "Maneerat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruz",
"given": "Cintia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Callery",
"given": "James J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Shivani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tuntipaiboontana",
"given": "Runch",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruabkontho",
"given": "Varaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ngernseng",
"given": "Thatsanun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tubprasert",
"given": "Jaruwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdad",
"given": "Mohammad Yazid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keayarsa",
"given": "Srisuda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madmanee",
"given": "Wanassanan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguiar",
"given": "Renato S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Franciele M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Pongtorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hanboonkunupakarn",
"given": "Borimas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poovorawan",
"given": "Kittiyod",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imwong",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taylor",
"given": "Walter R. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Vasin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chotivanich",
"given": "Kesinee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pukrittayakamee",
"given": "Sasithon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dondorp",
"given": "Arjen M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Day",
"given": "Nicholas P. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Mauro M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piyaphanee",
"given": "Watcharapong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phumratanaprapin",
"given": "Weerapong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "White",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the PLATCOV Collaborative Group",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T11:02:18Z",
"timestamp": 1705316538000
},
"deposited": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T23:01:47Z",
"timestamp": 1705359707000
},
"funder": [
{
"DOI": "10.13039/100010269",
"award": [
"Other",
"Other",
"Other",
"223195/Z/21/Z",
"Other",
"Other",
"Other",
"223195/Z/21/Z",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other",
"Other"
],
"doi-asserted-by": "publisher",
"name": "Wellcome Trust"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
16
]
],
"date-time": "2024-01-16T00:15:34Z",
"timestamp": 1705364134427
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T00:00:00Z",
"timestamp": 1705276800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T00:00:00Z",
"timestamp": 1705276800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-023-08835-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-023-08835-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-023-08835-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
1,
15
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
15
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1128/AAC.46.4.977-981.2002",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "977",
"journal-title": "Antimicrob Agents Chemother",
"key": "8835_CR1",
"unstructured": "Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–81.",
"volume": "46",
"year": "2002"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "446",
"journal-title": "Antiviral Res",
"key": "8835_CR2",
"unstructured": "Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–54.",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"author": "D Sissoko",
"doi-asserted-by": "publisher",
"first-page": "e1001967",
"journal-title": "PLoS Med",
"key": "8835_CR3",
"unstructured": "Sissoko D, Laouenan C, Folkesson E, M’Lebing AB, Beavogui AH, Baize S, et al. Experimental treatment with favipiravir for ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13:e1001967.",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "M Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res",
"key": "8835_CR4",
"unstructured": "Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.034",
"author": "Q Wang",
"doi-asserted-by": "publisher",
"first-page": "417",
"journal-title": "Cell",
"key": "8835_CR5",
"unstructured": "Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, et al. Structural basis for RNA replication by the SARS-CoV-2 polymerase. Cell. 2020;182:417–28.",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkaa171",
"author": "EA Coomes",
"doi-asserted-by": "publisher",
"first-page": "2013",
"journal-title": "J Antimicrob Chemother",
"key": "8835_CR6",
"unstructured": "Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–4.",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01739-09",
"author": "K Sleeman",
"doi-asserted-by": "publisher",
"first-page": "2517",
"journal-title": "Antimicrob Agents Chemother",
"key": "8835_CR7",
"unstructured": "Sleeman K, Mishin VP, Deyde VM, Furuta Y, Klimov AI, Gubareva LV. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 A(H1N1) viruses. Antimicrob Agents Chemother. 2010;54:2517–24.",
"volume": "54",
"year": "2010"
},
{
"DOI": "10.1073/pnas.2014441117",
"author": "SJF Kaptein",
"doi-asserted-by": "publisher",
"first-page": "26955",
"journal-title": "Proc Natl Acad Sci USA",
"key": "8835_CR8",
"unstructured": "Kaptein SJF, Jacobs S, Langendries L, Seldeslachts L, Ter Horst S, Liesenborghs L, et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci USA. 2020;117:26955–65.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"author": "JS Driouich",
"doi-asserted-by": "publisher",
"first-page": "1735",
"journal-title": "Nat Commun",
"key": "8835_CR9",
"unstructured": "Driouich JS, Cochin M, Lingas G, Moureau G, Touret F, Petit PR, et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat Commun. 2021;12:1735.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S349241",
"author": "PK Reddy",
"doi-asserted-by": "publisher",
"first-page": "4551",
"journal-title": "Int J Gen Med",
"key": "8835_CR10",
"unstructured": "Reddy PK, Patil S, Khobragade A, Balki A, Raj A, Kalikar M, et al. Evaluation of the safety and efficacy of favipiravir in adult Indian patients with mild-to-moderate COVID-19 in a real-world setting. Int J Gen Med. 2022;15:4551–63.",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1097/MD.0000000000031681",
"author": "T Sitasuwan",
"doi-asserted-by": "publisher",
"first-page": "e31681",
"journal-title": "Medicine (Baltimore)",
"key": "8835_CR11",
"unstructured": "Sitasuwan T, Phisalprapa P, Srivanichakorn W, Washirasaksiri C, Auesomwang C, Tinmanee R, et al. Early antiviral and supervisory dexamethasone treatment improve clinical outcomes of nonsevere COVID-19 patients. Medicine (Baltimore). 2022;101:e31681.",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"author": "Q Cai",
"doi-asserted-by": "publisher",
"first-page": "1192",
"issue": "10",
"journal-title": "Engineering",
"key": "8835_CR12",
"unstructured": "Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020;6(10):1192–8.",
"volume": "6",
"year": "2020"
},
{
"key": "8835_CR13",
"unstructured": "Doi Y, Ishihara T, Banno S, Ando M, Kondo M. Favipiravir Observational Study. Favipiravir for symptomatic COVID-19: a nationwide observational cohort study. J Infect Chemother. 2022:S1341–321X(22)00291–4."
},
{
"DOI": "10.55519/JAMC-03-10305",
"author": "B Abdulrahman",
"doi-asserted-by": "publisher",
"first-page": "397",
"issue": "3",
"journal-title": "J Ayub Med Coll Abbottabad",
"key": "8835_CR14",
"unstructured": "Abdulrahman B, Mady A, Odat MA, Tayar AA, Rana MA, Alharthy A, et al. Favipiravir efficacy and safety for the treatment of severe coronavirus disease 2019: a retrospective study. J Ayub Med Coll Abbottabad. 2022;34(3):397–402.",
"volume": "34",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"author": "R Sirijatuphat",
"doi-asserted-by": "publisher",
"first-page": "2197",
"journal-title": "Emerg Microbes Infect",
"key": "8835_CR15",
"unstructured": "Sirijatuphat R, Manosuthi W, Niyomnaitham S, Owen A, Copeland KK, Charoenpong L, et al. Early treatment of Favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study. Emerg Microbes Infect. 2022;11:2197–206.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1128/AAC.01897-20",
"author": "Y Doi",
"doi-asserted-by": "publisher",
"first-page": "e01897",
"journal-title": "Antimicrob Agents Chemother.",
"key": "8835_CR16",
"unstructured": "Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. Prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64:e01897–20.",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.008",
"author": "F Khamis",
"doi-asserted-by": "publisher",
"first-page": "538",
"journal-title": "Int J Infect Dis",
"key": "8835_CR17",
"unstructured": "Khamis F, Al Naabi H, Al Lawati A, Ambusaidi Z, Al Sharji M, Al Barwani U, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538–43.",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1176",
"author": "AA Ivashchenko",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "8835_CR18",
"unstructured": "Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase ii/iii multicenter randomized clinical trial. Clin Infect Dis. 2021;73:531–4.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.107702",
"doi-asserted-by": "crossref",
"key": "8835_CR19",
"unstructured": "Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97:107702."
},
{
"DOI": "10.1016/j.intimp.2021.107522",
"author": "M Solaymani-Dodaran",
"doi-asserted-by": "publisher",
"first-page": "107522",
"journal-title": "Int Immunopharmacol",
"key": "8835_CR20",
"unstructured": "Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, et al. Safety and efficacy of Favipiravir in moderate to severe SARS-CoV-2 pneumonia. Int Immunopharmacol. 2021;95:107522.",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"author": "ZF Udwadia",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "8835_CR21",
"unstructured": "Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71.",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-08794-w",
"author": "M Al Qahtani",
"doi-asserted-by": "publisher",
"first-page": "4925",
"issue": "1",
"journal-title": "Sci Rep",
"key": "8835_CR22",
"unstructured": "Al Qahtani M, Kumar N, Aljawder D, Abdulrahman A, Mohamed MW, Alnashaba F, et al. Randomized controlled trial of favipiravir, hydroxychloroquine, and standard care in patients with mild/moderate COVID-19 disease. Sci Rep. 2022;12(1):4925.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27724",
"author": "M Hassaniazad",
"doi-asserted-by": "publisher",
"first-page": "3184",
"journal-title": "J Med Virol",
"key": "8835_CR23",
"unstructured": "Hassaniazad M, Farshidi H, Gharibzadeh A, Bazram A, Khalili E, Noormandi A, Fathalipour M. Efficacy and safety of favipiravir plus interferon-beta versus lopinavir/ritonavir plus interferon-beta in moderately ill patients with COVID-19: a randomized clinical trial. J Med Virol. 2022;94:3184–91.",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2022.101703",
"author": "JH McMahon",
"doi-asserted-by": "publisher",
"first-page": "101703",
"journal-title": "EClinicalMedicine",
"key": "8835_CR24",
"unstructured": "McMahon JH, Lau JSY, Coldham A, Roney J, Hagenauer M, Price S, et al. Favipiravir in early symptomatic COVID-19, a randomised placebo-controlled trial. EClinicalMedicine. 2022;54:101703.",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.1004120",
"author": "DM Lowe",
"doi-asserted-by": "publisher",
"first-page": "e1004120",
"journal-title": "PLoS Med",
"key": "8835_CR25",
"unstructured": "Lowe DM, Brown LK, Chowdhury K, Davey S, Yee P, Ikeji F, et al. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): a randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med. 2022;19:e1004120.",
"volume": "19",
"year": "2022"
},
{
"author": "Y Golan",
"first-page": "ciac712",
"journal-title": "Clin Infect Dis.",
"key": "8835_CR26",
"unstructured": "Golan Y, Campos JAS, Woolson R, Cilla D, Hanabergh R, Gonzales-Rojas Y, et al. Favipiravir in patients with early mild-to-moderate COVID-19: a randomized controlled trial. Clin Infect Dis. 2022;6:ciac712.",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2021.12.026",
"author": "M Bosaeed",
"doi-asserted-by": "publisher",
"first-page": "602",
"journal-title": "Clin Microbiol Infect",
"key": "8835_CR27",
"unstructured": "Bosaeed M, Alharbi A, Mahmoud E, Alrehily S, Bahlaq M, Gaifer Z, et al. Efficacy of favipiravir in adults with mild COVID-19: a randomized, double-blind, multicentre, placebo-controlled clinical trial. Clin Microbiol Infect. 2022;28:602–8.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciab962",
"author": "CH Chuah",
"doi-asserted-by": "publisher",
"first-page": "e432",
"journal-title": "Clin Infect Dis",
"key": "8835_CR28",
"unstructured": "Chuah CH, Chow TS, Hor CP, Cheng JT, Ker HB, Lee HG, et al. Efficacy of early treatment with favipiravir on disease progression among high-risk patients with coronavirus disease 2019 (COVID-19): a randomized open-label clinical trial. Clin Infect Dis. 2022;75:e432–9.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac312",
"author": "M Holubar",
"doi-asserted-by": "publisher",
"first-page": "1883",
"journal-title": "Clin Infect Dis",
"key": "8835_CR29",
"unstructured": "Holubar M, Subramanian A, Purington N, Hedlin H, Bunning B, Walter KS, et al. Favipiravir for treatment of outpatients with asymptomatic or uncomplicated coronavirus disease 2019: a double-blind, randomized, placebo-controlled, phase 2 trial. Clin Infect Dis. 2022;75:1883–92.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1002/em.22471",
"author": "MD Waters",
"doi-asserted-by": "publisher",
"first-page": "37",
"journal-title": "Environ Mol Mutagen",
"key": "8835_CR30",
"unstructured": "Waters MD, Warren S, Hughes C, Lewis P, Zhang F. Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir. Environ Mol Mutagen. 2022;63:37–63.",
"volume": "63",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00493-0",
"doi-asserted-by": "publisher",
"key": "8835_CR31",
"unstructured": "Schilling WHK, Jittamala P, Watson JA, Boyd S, Luvira V, Siripoon T, et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect Dis. 2023:S1473-3099(23)00493-0. https://doi.org/10.1016/S1473-3099(23)00493-0. Epub ahead of print. Erratum in: Lancet Infect Dis. 2023;23(12):e511."
},
{
"DOI": "10.1002/cpt.1844",
"author": "YX Du",
"doi-asserted-by": "publisher",
"first-page": "242",
"journal-title": "Clin Pharmacol Ther",
"key": "8835_CR32",
"unstructured": "Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;108:242–7.",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "8835_CR33",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386:509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "8835_CR34",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1371/journal.pntd.0005389",
"author": "TH Nguyen",
"doi-asserted-by": "publisher",
"first-page": "e0005389",
"journal-title": "PLoS Negl Trop Dis",
"key": "8835_CR35",
"unstructured": "Nguyen TH, Guedj J, Anglaret X, Laouénan C, Madelain V, Taburet AM, et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017;11:e0005389.",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.7554/eLife.83201",
"doi-asserted-by": "publisher",
"key": "8835_CR36",
"unstructured": "Schilling WHK, Jittamala P, Watson JA, Ekkapongpisit M, Siripoon T, Ngamprasertchai T, et al. Pharmacometrics of high-dose ivermectin in early COVID-19 from an open label, randomized, controlled adaptive platform trial (PLATCOV). Elife. 2023;12:e83201. https://doi.org/10.7554/eLife.83201."
},
{
"DOI": "10.1056/NEJMoa2115869",
"author": "G Reis",
"doi-asserted-by": "publisher",
"first-page": "1721",
"journal-title": "N Engl J Med",
"key": "8835_CR37",
"unstructured": "Reis G, Silva EASM, Silva DCM, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with ivermectin among patients with Covid-19. N Engl J Med. 2022;386:1721–31.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad275",
"author": "P Jittamala",
"doi-asserted-by": "publisher",
"first-page": "1318",
"issue": "10",
"journal-title": "J Infect Dis",
"key": "8835_CR38",
"unstructured": "Jittamala P, Schilling WHK, Watson JA, Luvira V, Siripoon T, Ngamprasertchai T, et al. Clinical antiviral efficacy of remdesivir in COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV). J Infect Dis. 2023;228(10):1318–25. https://doi.org/10.1093/infdis/jiad275.",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "8835_CR39",
"unstructured": "Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–15.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "8835_CR40",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–51.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"author": "MP O'Brien",
"doi-asserted-by": "publisher",
"first-page": "1184",
"journal-title": "N Engl J Med.",
"key": "8835_CR41",
"unstructured": "O’Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. Covid-19 phase 3 prevention trial team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–95.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"author": "WA Fischer 2nd",
"doi-asserted-by": "publisher",
"first-page": "eabl7430",
"journal-title": "Sci Transl Med",
"key": "8835_CR42",
"unstructured": "Fischer WA 2nd, Eron JJ Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"author": "CC Butler",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Lancet",
"key": "8835_CR43",
"unstructured": "Butler CC, Hobbs DR, Gbinigie OA, Rahman NM, Hayward G, Richards DB, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401:281–93.",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1128/aac.00192-22",
"author": "JA Watson",
"doi-asserted-by": "publisher",
"first-page": "e0019222",
"journal-title": "Antimicrob Agents Chemother",
"key": "8835_CR44",
"unstructured": "Watson JA, Kissler SM, Day NPJ, Grad YH, White NJ. Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies. Antimicrob Agents Chemother. 2022;66:e0019222.",
"volume": "66",
"year": "2022"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-023-08835-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases"
],
"subtitle": [],
"title": "Clinical antiviral efficacy of favipiravir in early COVID-19 (PLATCOV): an open-label, randomised, controlled, adaptive platform trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}