Pulmonary delivery of favipiravir inhalation solution for COVID-19 treatment: in vitro characterization, stability, in vitro cytotoxicity, and antiviral activity using real time cell analysis
et al., Drug Delivery, doi:10.1080/10717544.2022.2118398, Sep 2022
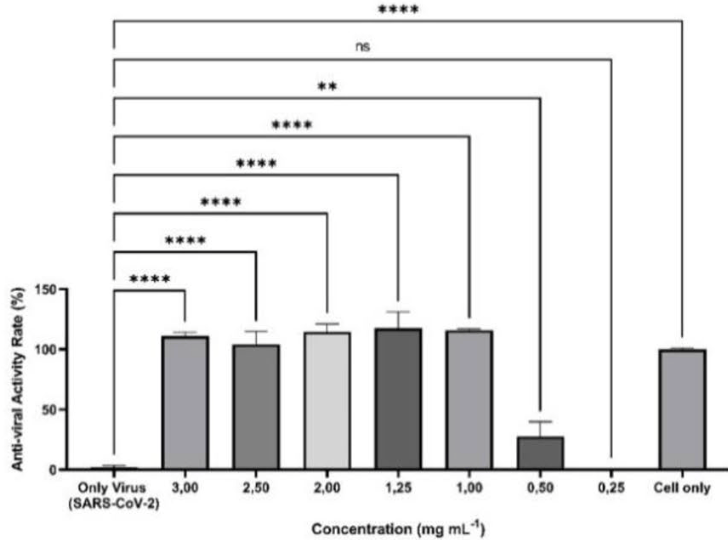
In vitro study of a favipiravir solution for inhalation, showing effective antiviral activity for SARS-CoV-2 with significantly lower doses than typically used with oral administration.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
4 preclinical studies support the efficacy of favipiravir for COVID-19:
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
14.
Mihaljevic et al., DNA damage in peripheral blood lymphocytes of severely ill COVID-19 patients in relation to inflammatory markers and parameters of hemostasis, Mutagenesis, doi:10.1093/mutage/geac011.
15.
Unal et al., Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2, bioRxiv, doi:10.1101/2022.01.11.475889.
Yildiz Pekoz et al., 5 Sep 2022, peer-reviewed, 14 authors.
Contact: aycay@istanbul.edu.tr.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Pulmonary delivery of favipiravir inhalation solution for COVID-19 treatment: in vitro characterization, stability, in vitro cytotoxicity, and antiviral activity using real time cell analysis
Drug Delivery, doi:10.1080/10717544.2022.2118398
Favipiravir, an RNA-dependent RNA polymerase (RdRp) inhibitor, is used to treat patients infected with influenza virus and most recently with SARS-CoV-2. However, poor accumulation of favipiravir in lung tissue following oral administration has required an alternative method of administration that directly targets the lungs. In this study, an inhalation solution of favipiravir at a concentration of 2 mg mL −1 was developed and characterized for the first time. The chemical stability of inhaled favipiravir solution in two different media, phosphate buffer saline (PBS) and normal saline (NS), was investigated under different conditions: 5 ± 3 °C, 25 ± 2 °C/60% RH ± 5% RH, and 40 ± 2 °C/75% RH ± 5% RH; in addition to constant light exposure. As a result, favipiravir solution in PBS revealed superior stability over 12 months at 5 ± 3 °C. Antiviral activity of favipiravir was assessed at the concentrations between 0.25 and 3 mg mL −1 with real time cell analyzer on Vero-E6 that were infected with SARS-CoV-2/B.1.36. The optimum concentration was found to be 2 mg mL −1 , where minimum toxicity and sufficient antiviral activity was observed. Furthermore, cell viability assay against Calu-3 lung epithelial cells confirmed the biocompatibility of favipiravir at concentrations up to 50 μM (7.855 mg mL −1 ). The in vitro aerodynamic profiles of the developed inhaled favipiravir formulation, when delivered with soft-mist inhaler indicated good lung targeting properties. These results suggest that favipiravir solution prepared with PBS could be considered as a suitable and promising inhalation formulation for pulmonary delivery in the treatment of patients with COVID-19.
References
Alamer, Alrashed, Alfaifi, Effectiveness and safety of favipiravir compared to supportive care in moderately to critically ill COVID-19 patients: a retrospective study with propensity score matching sensitivity analysis, Curr Med Res Opin
Almoosa, Saad, Qara, Favipiravir versus standard of care in patients with severe COVID-19 infections: a retrospective comparative study, J Infect Public Health
Amirav, Newhouse, Transmission of coronavirus by nebulizer: a serious, underappreciated risk, CMAJ
Andersen, Rambaut, Lipkin, The proximal origin of SARS-CoV-2, Nat Med
Anderson, Use of Respimat Soft Mist inhaler in COPD patients, Int J Chron Obstruct Pulmon Dis
Brand, Hederer, Austen, Higher lung deposition with Respimat® Soft Mist TM Inhaler than HFA-MDI in COPD patients with poor technique, Int J COPD
Charretier, Saulnier, Benair, Robust real-time cell analysis method for determining viral infectious titers during development of a viral vaccine production process, J Virol Methods
Ciciliani, Denny, Langguth, Lung deposition using the Respimat® Soft Mist TM inhaler mono and fixed-dose combination therapies: an in vitro/in silico analysis, COPD J Chronic Obstr Pulm Dis
Deng, Yang, Yang, Evaluation of favipiravir in the treatment of COVID-19 based on the real-world, Expert Rev anti Infect Ther
Du, Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin Pharmacol Ther
Durdagi, Avsar, Orhan, The neutralization effect of montelukast on SARS-CoV-2 is shown by multiscale in silico simulations and combined in vitro studies, Mol Ther
Elezkurtaj, Greuel, Ihlow, Causes of death and comorbidities in hospitalized patients with COVID-19, Nat Sci Rep
Erelel, Kaskal, Akbal-Dagistan, Early effects of low molecular weight heparin therapy with soft-mist inhaler for COVID-19-induced hypoxemia: a phase IIb trial, Pharmaceutics
Fang, Ye, Wang, Real-time monitoring of flavivirus induced cytopathogenesis using cell electric impedance technology, J Virol Methods
Fazekas, Funk, Klobassa, Evaluation of 36 formulas for calculating plasma osmolality, Intensive Care Med
Fischer, Widdicombe, Mechanisms of acid and base secretion by the airway epithelium, J Membr Biol
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Ghasemnejad-Berenji, Pashapour, Favipiravir and COVID-19: a simplified summary, Drug Res (Stuttg)
Hassanipour, Arab-Zozani, Amani, The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials, Sci Rep
Jeon, Ko, Lee, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob. Agents Chemother
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Kow, Ramachandram, Hasan, Future of antivirals in COVID-19: the case of favipiravir, Int Immunopharmacol
Kumar, Jayanti, Johnson, Metabolism and disposition of the HIV-1 protease inhibitor lopinavir (ABT-378) given in combination with ritonavir in rats, dogs, and humans, Pharm Res
Leiner, Cipolla, Eicher, Soft mist sprays
Marzouk, Rezk, Gouda, Abdel-Megied, A novel stability-indicating HPLC-DAD method for determination of favipiravir, a potential antiviral drug for COVID-19 treatment; application to degradation kinetic studies and in-vitro dissolution profiling, Microchem J
Newman, Brown, Steed, Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines * comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices, Chest
Pilcer, Amighi, Formulation strategy and use of excipients in pulmonary drug delivery, Int J Pharm
Pitcairn, Reader, Newman, Deposition of corticosteroid aerosol in the human lung by respimat® Soft Mist TM inhaler compared to deposition by metered dose inhaler or by Turbuhaler® dry powder inhaler, J. Aerosol Med
Sahin, Halje, Uzun, Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment, J Pharm Sci
Shannon, Selisko, Le, Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis, Nat Commun
Shiraki, Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol Ther
Takayama, In vitro and animal models for SARS-CoV-2 research, Trends Pharmacol Sci
Taşlı, Gönen, Kırbaş, Preclinical studies on convalescent human immune plasma-derived exosome: omics and antiviral properties to SARS-CoV-2, Front Immunol
Teng, Kuang, Wang, Zhang, Real-time cell analysis -a new method for dynamic, quantitative measurement of infectious viruses and antiserum neutralizing activity, J Virol Methods
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Vemuri, Gundla, Konduru, SARS-CoV-2) degradation impurities: Identification and route of degradation mechanism in the finished solid dosage form using LC/LC-MS method, Biomed Chromatogr
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Lung tissue distribution of drugs as a key factor for COVID-19 treatment, Br J Pharmacol
Wiersinga, Rhodes, Cheng, Transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA
Zhang, Li, Zhang, Identifying airborne transmission as the dominant route for the spread of COVID-19, Proc Natl Acad Sci
Zhu, Li, Pang, Recent insights for the emerging COVID-19: drug discovery, therapeutic options and vaccine development, Asian J Pharm Sci
Zost, Gilchuk, Chen, Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein, Nat Med
DOI record:
{
"DOI": "10.1080/10717544.2022.2118398",
"ISSN": [
"1071-7544",
"1521-0464"
],
"URL": "http://dx.doi.org/10.1080/10717544.2022.2118398",
"alternative-id": [
"10.1080/10717544.2022.2118398"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=idrd20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=idrd20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-07-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2022-08-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2022-08-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2022-09-05"
}
],
"author": [
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
}
],
"family": "Yildiz Pekoz",
"given": "Ayca",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
}
],
"family": "Akbal Dagistan",
"given": "Ozlem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
}
],
"family": "Fael",
"given": "Hanan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
}
],
"family": "Culha",
"given": "Meltem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
},
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istinye University, Istanbul, Türkiye"
}
],
"family": "Erturk",
"given": "Aybige",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Istanbul University, Istanbul, Türkiye"
}
],
"family": "Basarir",
"given": "Nur Sena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Pharmacy, Department of Pharmaceutical Technology, Trakya University, Istanbul, Türkiye"
}
],
"family": "Sahin",
"given": "Gokben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Biotechnology (Marmara Research Center (MRC)), TUBITAK Marmara Research Center-MRC, Life Sciences, Kocaeli, Türkiye"
}
],
"family": "Serhatli",
"given": "Muge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Biotechnology (Marmara Research Center (MRC)), TUBITAK Marmara Research Center-MRC, Life Sciences, Kocaeli, Türkiye"
},
{
"name": "Molecular Biology and Genetics, Institute of Natural and Applied Sciences, Gebze Technical University, Kocaeli, Türkiye"
}
],
"family": "Cakirca",
"given": "Gamze",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Biotechnology (Marmara Research Center (MRC)), TUBITAK Marmara Research Center-MRC, Life Sciences, Kocaeli, Türkiye"
},
{
"name": "Hamidiye Faculty of Medicine, Department of Basic Medical Sciences, Medical Biology, University of Health Sciences, Istanbul, Türkiye"
}
],
"family": "Tekin",
"given": "Saban",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Basic Medical Sciences, Department of Physiology, Marmara University, Istanbul, Türkiye"
}
],
"family": "Sen",
"given": "Leyla Semiha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Basic Medical Sciences, Department of Physiology, Marmara University, Istanbul, Türkiye"
}
],
"family": "Sevim",
"given": "Mustafa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Department of Infectious Diseases, Marmara University, Istanbul, Türkiye."
}
],
"family": "Mulazimoglu Durmusoglu",
"given": "Lutfiye",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine, Basic Medical Sciences, Department of Physiology, Marmara University, Istanbul, Türkiye"
}
],
"family": "Yegen",
"given": "Berrak C.",
"sequence": "additional"
}
],
"container-title": "Drug Delivery",
"container-title-short": "Drug Delivery",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
5
]
],
"date-time": "2022-09-05T17:30:24Z",
"timestamp": 1662399024000
},
"deposited": {
"date-parts": [
[
2022,
9,
5
]
],
"date-time": "2022-09-05T17:30:33Z",
"timestamp": 1662399033000
},
"indexed": {
"date-parts": [
[
2022,
11,
9
]
],
"date-time": "2022-11-09T15:08:03Z",
"timestamp": 1668006483537
},
"is-referenced-by-count": 1,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
5
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
5
]
],
"date-time": "2022-09-05T00:00:00Z",
"timestamp": 1662336000000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/10717544.2022.2118398",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "2846-2854",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2022,
9,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1080/03007995.2021.1920900",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1016/j.jiph.2021.08.022",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1503/cmaj.75066",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"DOI": "10.1038/s41591-020-0820-9",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"author": "Anderson P.",
"first-page": "251",
"journal-title": "Int J Chron Obstruct Pulmon Dis",
"key": "CIT0005",
"volume": "1",
"year": "2006"
},
{
"DOI": "10.1080/17425247.2020.1811225",
"doi-asserted-by": "publisher",
"key": "CIT0006"
},
{
"DOI": "10.2147/COPD.S3930",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1016/j.jviromet.2017.11.002",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1080/15412555.2020.1853091",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.1080/14787210.2022.2012155",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.1002/cpt.1844",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.1016/j.ymthe.2021.10.014",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1038/s41598-021-82862-5",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"DOI": "10.3390/pharmaceutics13111768",
"doi-asserted-by": "publisher",
"key": "CIT0014"
},
{
"author": "European Directorate for the Quality of Medicines and Healthcare (EDQM)",
"first-page": "287",
"key": "CIT0015",
"volume-title": "Section 2.9.18—Preparations for inhalation: aerodynamic assessment of fine particles. European Pharmacopeia",
"year": "2009"
},
{
"DOI": "10.1016/j.jviromet.2011.02.013",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1007/s00134-012-2691-0",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.1007/s00232-006-0861-0",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.1055/a-1296-7935",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.1128/AAC.00819-20",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"doi-asserted-by": "publisher",
"key": "CIT0023"
},
{
"DOI": "10.1016/j.intimp.2021.108455",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1023/B:PHAM.0000041457.64638.8d",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"author": "Leiner S",
"first-page": "493",
"key": "CIT0026",
"volume": "35",
"volume-title": "Pharmaceutical inhalation aerosol technology",
"year": "2017"
},
{
"DOI": "10.1016/j.microc.2021.106917",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"DOI": "10.1378/chest.113.4.957",
"doi-asserted-by": "publisher",
"key": "CIT0028"
},
{
"DOI": "10.1016/j.ijpharm.2010.03.017",
"doi-asserted-by": "publisher",
"key": "CIT0029"
},
{
"DOI": "10.1089/jam.2005.18.264",
"doi-asserted-by": "publisher",
"key": "CIT0030"
},
{
"author": "Sahin G",
"first-page": "861668",
"journal-title": "J Pharm Sci",
"key": "CIT0031",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-18463-z",
"doi-asserted-by": "publisher",
"key": "CIT0032"
},
{
"DOI": "10.1016/j.pharmthera.2020.107512",
"doi-asserted-by": "publisher",
"key": "CIT0033"
},
{
"DOI": "10.1016/j.tips.2020.05.005",
"doi-asserted-by": "publisher",
"key": "CIT0034"
},
{
"DOI": "10.3389/fimmu.2022.824378",
"doi-asserted-by": "publisher",
"key": "CIT0035"
},
{
"DOI": "10.1016/j.jviromet.2013.06.034",
"doi-asserted-by": "publisher",
"key": "CIT0036"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "CIT0037"
},
{
"DOI": "10.1002/bmc.5363",
"doi-asserted-by": "publisher",
"key": "CIT0038"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "CIT0039"
},
{
"DOI": "10.1111/bph.15102",
"doi-asserted-by": "publisher",
"key": "CIT0040"
},
{
"DOI": "10.1001/jama.2020.12839",
"doi-asserted-by": "publisher",
"key": "CIT0041"
},
{
"DOI": "10.1073/pnas.2009637117",
"doi-asserted-by": "publisher",
"key": "CIT0042"
},
{
"DOI": "10.1016/j.ajps.2020.06.001",
"doi-asserted-by": "publisher",
"key": "CIT0043"
},
{
"DOI": "10.1038/s41591-020-0998-x",
"doi-asserted-by": "publisher",
"key": "CIT0044"
}
],
"reference-count": 44,
"references-count": 44,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/10717544.2022.2118398"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmaceutical Science",
"General Medicine"
],
"subtitle": [],
"title": "Pulmonary delivery of favipiravir inhalation solution for COVID-19 treatment: <i>in vitro</i> characterization, stability, <i>in vitro</i> cytotoxicity, and antiviral activity using real time cell analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "29"
}