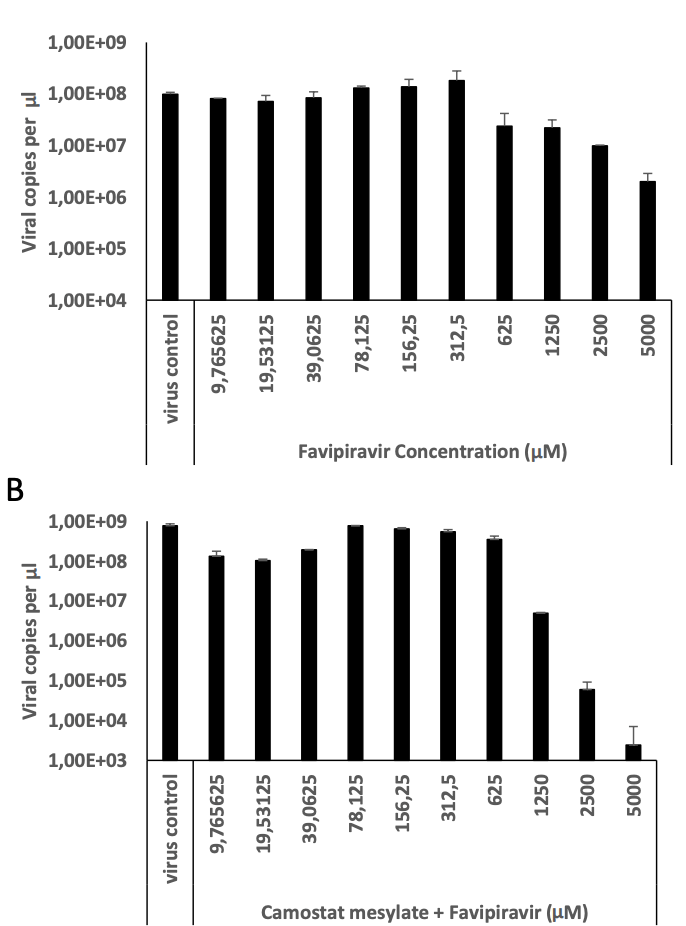
Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2
et al., bioRxiv, doi:10.1101/2022.01.11.475889, Jan 2022
In vitro and in silico study showing that the combination of favipiravir and umifenovir or camostat mesylate has greater antiviral efficacy than single drug treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
4 preclinical studies support the efficacy of favipiravir for COVID-19:
Study covers favipiravir, camostat, and TMPRSS2 inhibitors.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
14.
Mihaljevic et al., DNA damage in peripheral blood lymphocytes of severely ill COVID-19 patients in relation to inflammatory markers and parameters of hemostasis, Mutagenesis, doi:10.1093/mutage/geac011.
15.
Unal et al., Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2, bioRxiv, doi:10.1101/2022.01.11.475889.
Unal et al., 12 Jan 2022, preprint, 10 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2
doi:10.1101/2022.01.11.475889
Since the first cases the coronavirus disease caused by SARS-CoV-2 (COVID-19) reported in December 2019, worldwide continuous efforts have been placed both for the prevention and treatment of this infectious disease. As new variants of the virus emerge, the need for an effective antiviral treatment continues. The concept of preventing SARS-CoV-2 on both pre-entry and post-entry stages has not been much studied. Therefore, we compared the antiviral activities of three antiviral drugs which have been currently used in the clinic. In silico docking analyses and in vitro viral infection in Vero E6 cells were performed to delineate their antiviral effectivity when used alone or in combination. Both in silico and in vitro results suggest that the combinatorial treatment by favipiravir and umifenovir or camostat mesylate has more antiviral activity against SARS-CoV-2 rather than single drug treatment. These results suggest that inhibiting both viral entry and viral replication at the same time is much more effective for the antiviral treatment of SARS-CoV-2.
which have different mechanism of action can eventuate to an effective antiviral thearpy for SARS-CoV-2 infection. Preclincal and clinical studies are ongoing for drug combinations to combat SARS-CoV-2. The concept of preventing the virus on both pre-entry and post-entry stages has not been previously studied. Favipiravir is broad-spectrum antiviral pro-dug which inhibits viral replication by influencing the activiy of RdRp (Delang et al., 2018) . Favipiravir enters cell through cell membrane and phophoribosylated by Hypoxanthine Guanine Phosphoribosyltransferase (HGPRT) to become Favipiravir-ribose-5'-monophosphate (Favipiravir-RMP) (Naesens et al., 2013) . Favipiravir-RMP turns into Favipiravir-ribose-5-diphosphate (Favipiravir-RDP) and Favipiravir-ribose-5'-triphosphate (Favipiravir-RTP) respectively by phosphorylation. Favipiravir-RTP competes with purine basespredominantly GTP-to influence viral RdRp mediated viral replication (Furuta et al., 2013) . Favipiravir-RTP can base pair with both cysteine and uracil base pairs. In our study, the antiviral activity of favipiravir and umifenovir or favipiravir and camostat mesylate combinations are much more effective than mono drug threapies. Doi et al. showed that combination of favipiravir and another TMPRSS2 inhibitor nafamostat mesylate showed promising results indicating combinatorial treatment inhibits both viral replication and viral entry (Doi et al., 2020) . Therefore, it is reasonable to target both viral entry..
References
Breining, Frølund, Højen, Gunst, Staerke et al., Camostat mesylate against SARS-CoV-2 and COVID-19-Rationale, dosing and safety, Basic \& Clin. Pharmacol. \& Toxicol, doi:10.1111/bcpt.13533
Cai, Yang, Liu, Chen, Shu et al., Experimental Treatment with Favipiravir COVID-19: An Open-Label Control Study, Eng, doi:10.1016/j.eng.2020.03.007
Chen, Zhang, Huang, Yin, Cheng et al., Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial, medRxiv, doi:10.1101/2020.03.17.20037432
Delang, Abdelnabi, Neyts, Favipiravir as a potential countermeasure against neglected and emerging RNA viruses, Antiviral Res, doi:10.1016/j.antiviral.2018.03.003
Deng, Li, Zeng, Liu, Li et al., Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study, J. Infect, doi:10.1016/j.jinf.2020.03.002
Doi, Ikeda, Hayase, Moriya, Morimura et al., Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series, Crit. Care, doi:10.1186/s13054-020-03078-z
Finkel, Mizrahi, Nachshon, Weingarten-Gabbay, Morgenstern et al., The coding capacity of SARS-CoV-2, Nature, doi:10.1038/s41586-020-2739-1
Frisch, Trucks, Schlegel, Scuseria, Robb et al., Gaussian G09. Gaussian Inc
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res, doi:10.1016/j.antiviral.2013.09.015
Gunst, Staerke, Pahus, Kristensen, Bodilsen et al., Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100849
Halboub, Al-Maweri, Al-Soneidar, COVID-19: A review of the proposed pharmacological treatments, Eur. J. Pharmacol
Hillen, Kokic, Farnung, Dienemann, Tegunov et al., Structure of replicating SARS-CoV-2 polymerase, Nature, doi:10.1038/s41586-020-2368-8
Hoffmann, Hofmann-Winkler, Smith, Krüger, Arora et al., Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Pöhlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Mol. Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hofmann-Winkler, Moerer, Alt-Epping, Bräuer, Büttner et al., Camostat Mesylate May Reduce Severity of Coronavirus Disease 2019 Sepsis: A First Observation, Crit. care Explor, doi:10.1097/CCE.0000000000000284
Huang, Guan, Yang, Grange, Le et al., Chloroquine, arbidol (umifenovir) or lopinavir/ritonavir as the antiviral monotherapy for COVID-19 patients: a retrospective cohort study, Res. Sq, doi:10.21203/rs.3.rs-24667/v1
Kitagawa, Arai, Iida, Mukai, Furukawa et al., A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID-19, Clin. Transl. Sci. n/a, doi:10.1111/cts.13052
Knoops, Kikkert, Van Den Worm, Zevenhoven-Dobbe, Van Der Meer et al., SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum, PLoS Biol, doi:10.1371/journal.pbio.0060226
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Letko, Marzi, Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses, Nat. Microbiol, doi:10.1038/s41564-020-0688-y
Li, Li, Wang, Poirier, Huang, Multiple Ligand Simultaneous Docking (MLSD) and Its Applications to Fragment Based Drug Design and Drug Repositioning DISSERTATION
Lian, Xie, Lin, Huang, Zhao et al., Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2020.04.026
Murgolo, Therien, Howell, Klein, Koeplinger et al., SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development, PLOS Pathog, doi:10.1371/journal.ppat.1009225
Naesens, Guddat, Keough, Van Kuilenburg, Meijer et al., Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir), Mol. Pharmacol, doi:10.1124/mol.113.087247
Pettersen, Goddard, Huang, Couch, Greenblatt et al., UCSF Chimera -A visualization system for exploratory research and analysis, J. Comput. Chem, doi:10.1002/jcc.20084
Pécheur, Lavillette, Alcaras, Molle, Boriskin et al., Biochemical mechanism of hepatitis C virus inhibition by the broad-spectrum antiviral arbidol, Biochemistry, doi:10.1021/bi700181j
Trott, Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem, doi:10.1002/jcc.21334
Uno, Camostat mesilate therapy for COVID-19, Intern. Emerg. Med, doi:10.1007/s11739-020-02345-9
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00468-6
Vankadari, Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105998
Wang, Cao, Zhang, Liu, Xu et al., The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro, Cell Discov, doi:10.1038/s41421-020-0169-8
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yuan, Pei, Lai, Binding Site Detection and Druggability Prediction of Protein Targets for Structure-Based Drug Design, Curr. Pharm. Des, doi:10.2174/1381612811319120019
DOI record:
{
"DOI": "10.1101/2022.01.11.475889",
"URL": "http://dx.doi.org/10.1101/2022.01.11.475889",
"abstract": "<jats:p>Since the first cases the coronavirus disease caused by SARS-CoV-2 (COVID-19) reported in December 2019, worldwide continuous efforts have been placed both for the prevention and treatment of this infectious disease. As new variants of the virus emerge, the need for an effective antiviral treatment continues. The concept of preventing SARS-CoV-2 on both pre-entry and post-entry stages has not been much studied. Therefore, we compared the antiviral activities of three antiviral drugs which have been currently used in the clinic. In silico docking analyses and in vitro viral infection in Vero E6 cells were performed to delineate their antiviral effectivity when used alone or in combination. Both in silico and in vitro results suggest that the combinatorial treatment by favipiravir and umifenovir or camostat mesylate has more antiviral activity against SARS-CoV-2 rather than single drug treatment. These results suggest that inhibiting both viral entry and viral replication at the same time is much more effective for the antiviral treatment of SARS-CoV-2.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
1,
12
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8607-5043",
"affiliation": [],
"authenticated-orcid": false,
"family": "Unal",
"given": "Mehmet Altay",
"sequence": "first"
},
{
"affiliation": [],
"family": "Besbinar",
"given": "Omur",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8423-751X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nazir",
"given": "Hasan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6274-3385",
"affiliation": [],
"authenticated-orcid": false,
"family": "Summak",
"given": "Gokce Yagmur",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7531-5080",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bayrakdar",
"given": "Fatma",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2329-7260",
"affiliation": [],
"authenticated-orcid": false,
"family": "Delogu",
"given": "Lucia Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taskin",
"given": "Tambay",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7494-3077",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ozkan",
"given": "Sibel Aysil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7816-6938",
"affiliation": [],
"authenticated-orcid": false,
"family": "Akcali",
"given": "Kamil Can",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yilmazer",
"given": "Acelya",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
13
]
],
"date-time": "2022-01-13T03:15:16Z",
"timestamp": 1642043716000
},
"deposited": {
"date-parts": [
[
2022,
1,
13
]
],
"date-time": "2022-01-13T03:15:17Z",
"timestamp": 1642043717000
},
"group-title": "Pharmacology and Toxicology",
"indexed": {
"date-parts": [
[
2022,
1,
13
]
],
"date-time": "2022-01-13T03:42:21Z",
"timestamp": 1642045341087
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
1,
12
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.01.11.475889",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
12
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
12
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Favipiravir, umifenovir and camostat mesylate: a comparative study against SARS-CoV-2"
],
"type": "posted-content"
}