Effect of Single High Dose Vitamin D Substitution in Hospitalized COVID-19 Patients with Vitamin D Deficiency on Length of Hospital Stay
et al., Biomedicines, doi:10.3390/biomedicines11051277, Apr 2023
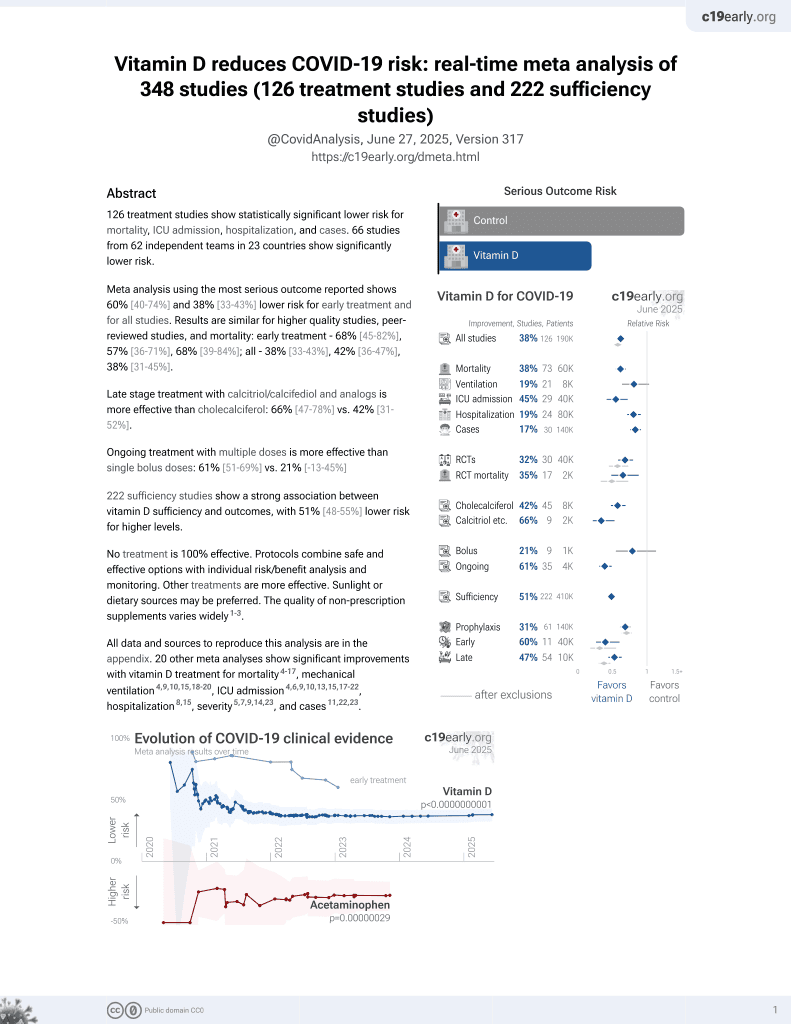
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT late stage patients showing no significant differences with the addition of single dose 140,000IU vitamin D treatment. All patients received vitamin D 800IU daily. There was a non-significant shorter length of stay for patients with vitamin D deficiency. The SF-12 mental score was significantly lower in the treatment group at baseline.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 34th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 116th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
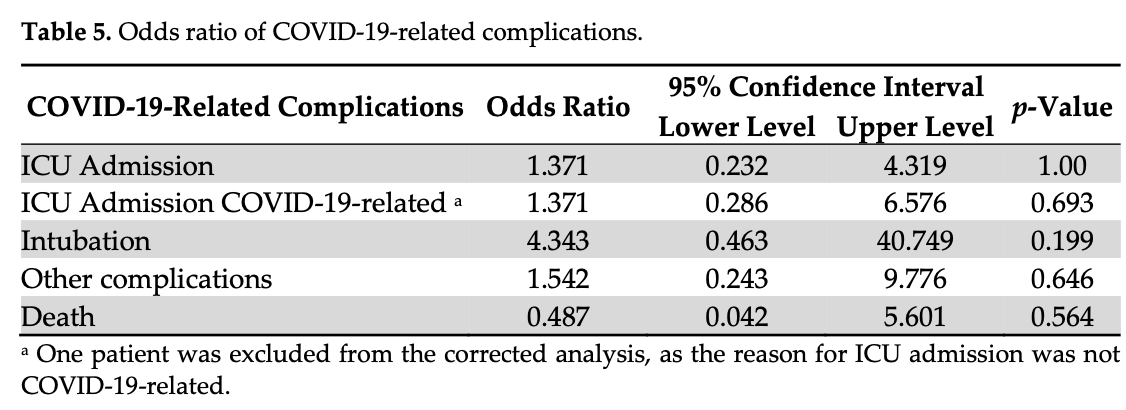
risk of death, 50.0% lower, RR 0.50, p = 0.56, treatment 1 of 39 (2.6%), control 2 of 39 (5.1%), NNT 39, odds ratio converted to relative risk.
|
|
risk of mechanical ventilation, 300.0% higher, RR 4.00, p = 0.20, treatment 4 of 39 (10.3%), control 1 of 39 (2.6%), odds ratio converted to relative risk.
|
|
risk of ICU admission, 32.1% higher, RR 1.32, p = 0.69, treatment 4 of 39 (10.3%), control 4 of 39 (10.3%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jaun et al., 25 Apr 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Switzerland, peer-reviewed, 14 authors, study period December 2020 - August 2021, average treatment delay 8.0 days, dosage 140,000IU single dose.
Effect of Single High Dose Vitamin D Substitution in Hospitalized COVID-19 Patients with Vitamin D Deficiency on Length of Hospital Stay
doi:10.3390/biomedicines11051277
Vitamin D and its role in the coronavirus-19 disease (COVID-19) pandemic has been controversially discussed, with inconclusive evidence about vitamin D3 (cholecalciferol) supplementation in COVID-19 patients. Vitamin D metabolites play an important role in the initiation of the immune response and can be an easily modifiable risk factor in 25-hydroxyvitamin D3 (25(OH)D3)-deficient patients. This is a multicenter, randomized, placebo-controlled doubleblind trial to compare the effect of a single high dose of vitamin D3 followed by treatment as usual (TAU) of daily vitamin D3 daily until discharge versus placebo plus TAU in hospitalized patients with COVID-19 and 25(OH)D3-deficiency on length hospital stay. We included 40 patients per group and did not observe a significant difference in the median length of hospital stay (6 days in both groups, p = 0.920). We adjusted the length of stay for COVID-19 risk factors (β = 0.44; 95% CI: −2.17-2.22), and center (β = 0.74; 95% CI: −1.25-2.73). The subgroup analysis in patients with severe 25(OH)D3-deficiency (<25 nmol/L) showed a non-significant reduction in the median length of hospital stay in the intervention group (5.5 vs. 9 days, p = 0.299). The competing risk model with death did not reveal significant differences between the group in the length of stay (HR = 0.96, 95% CI 0.62-1.48, p = 0.850). Serum 25(OH)D3 level increased significantly in the intervention group (mean change in nmol/L; intervention: +26.35 vs. control: -2.73, p < 0.001). The intervention with 140,000 IU vitamin D3 + TAU did not significantly shorten the length of hospital stay but was effective and safe for the elevation of serum 25(OH)D3 levels.
Supplementary Materials: The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11051277/s1, Table S1 : Visit procedure and collected information for each patient at different time points during the study. Table S2 : Co-Morbidities at time of randomization. Table S3 : Concomitant medication during hospitalization. Table S4 . Robust linear regression results presenting the effect of group. Intervention vs. Control on time to discharge (from randomization) (univariable and adjusted for COVID-19 risk factors, prognostic imbalances, and center effects. Table S5 : Mean SF-12 mental and SF-12 physical scores at randomization, 28 days and 90 days after randomization. Table S6 : Mixed-effects linear regression results on SF-12 mental and physical outcome with a time and group interaction term. Table S7 : Frequency of self-reported symptoms at day 5 after randomization. Table S8 : Mean vital signs at day 5 after randomization. Table S9 : Frequency of self-reported symptoms at day 10 after randomization. Table S10 : Mean vital signs at day 10 after randomization. Table S11 : Frequency of adverse and serious adverse events occurred during the trial per study group. Table S12 : Levels of calcium, PTH and phosphorus at time of discharge.
Conflicts of Interest: The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the..
References
Al-Jarallah, Rajan, Dashti, Al Saber, Pan et al., In-hospital mortality in SARS-CoV-2 stratified by serum 25-hydroxy-vitamin D levels: A retrospective study, J. Med. Virol, doi:10.1002/jmv.27133
Ali, Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19, J. Med. Virol, doi:10.1002/jmv.26097
Alsafar, Grant, Hijazi, Uddin, Alkaabi et al., COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents, Nutrients, doi:10.3390/nu13051714
Alsufiani, Alghamdi, Alshaibi, Khoja, Saif et al., A Single Vitamin D3 Bolus Supplementation Improves Vitamin D Status and Reduces Proinflammatory Cytokines in Healthy Females, Nutrients, doi:10.3390/nu14193963
Amrein, Sourij, Wagner, Holl, Pieber et al., Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study, Crit. Care, doi:10.1186/cc10120
Annweiler, Beaudenon, Gautier, Gonsard, Boucher et al., High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial, PLoS Med, doi:10.1371/journal.pmed.1003999
Annweiler, Beaudenon, Simon, Guenet, Otekpo et al., GERIA-COVID study group Vitamin D supplementation prior to or during COVID-19 associated with better 3-month survival in geriatric patients: Extension phase of the GERIA-COVID study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2021.105958
Annweiler, Corvaisier, Gautier, Dubée, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Apaydin, Can, Kizilgul, Beysel, Kan et al., The effects of single high-dose or daily low-dosage oral colecalciferol treatment on vitamin D levels and muscle strength in postmenopausal women, BMC Endocr. Disord, doi:10.1186/s12902-018-0277-8
Asghar, Yasmin, Dapke, Shah, Zafar et al., Evaluation of Vitamin-D Status and Its Association with Clinical Outcomes Among COVID-19 Patients in Pakistan, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.21-0577
Ataide, Carvalho Bastos, Vicente Matias, Skare, Freire De Carvalho, Safety and effectiveness of vitamin D mega-dose: A systematic review, Clin. Nutr. ESPEN, doi:10.1016/j.clnesp.2021.09.010
Brass, Mckay, Scott, Investigating an incidental finding of lymphopenia, BMJ, doi:10.1136/bmj.g1721
Cannata-Andía, Díaz-Sottolano, Fernández, Palomo-Antequera, Herrero-Puente et al., A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial, BMC Med, doi:10.1186/s12916-022-02290-8
Cannell, Vieth, Umhau, Holick, Grant et al., Epidemic influenza and vitamin D, Epidemiol. Infect, doi:10.1017/S0950268806007175
Carlberg, Haq, The concept of the personal vitamin D response index, Vitam. Defic. Hum. Health, doi:10.1016/j.jsbmb.2016.12.011
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Cava, Neri, Carbonelli, Riso, Carbone, Obesity pandemic during COVID-19 outbreak: Narrative review and future considerations, Clin. Nutr. Edinb. Scotl, doi:10.1016/j.clnu.2021.02.038
Cervero, López-Wolf, Casado, Novella-Mena, Ryan-Murua et al., Beneficial Effect of Short-Term Supplementation of High Dose of Vitamin D3 in Hospitalized Patients With COVID-19: A Multicenter, Single-Blinded, Prospective Randomized Pilot Clinical Trial, Front. Pharmacol, doi:10.3389/fphar.2022.863587
Charoenngam, Holick, Immunologic Effects of Vitamin D on Human Health and Disease, Nutrients, doi:10.3390/nu12072097
Clark, Waters, Stanfill, Elevated liver function tests in COVID-19: Causes, clinical evidence, and potential treatments, Nurse Pract
Da Rocha, Atallah, Aldrighi, Pires, Dos Santos Puga et al., Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review, Int. J. Clin. Pract, doi:10.1111/ijcp.14649
Davoudi, Najafi, Aarabi, Tayebi, Nikaeen et al., Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection, BMC Infect. Dis, doi:10.1186/s12879-021-06168-7
De Niet, Trémège, Coffiner, Rousseau, Calmes et al., Positive Effects of Vitamin D Supplementation in Patients Hospitalized for COVID-19: A Randomized, Double-Blind, Placebo-Controlled Trial, Nutrients, doi:10.3390/nu14153048
Dessie, Zewotir, Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients, BMC Infect. Dis, doi:10.1186/s12879-021-06536-3
Elamir, Amir, Lim, Rana, Lopez et al., A randomized pilot study using calcitriol in hospitalized COVID-19 patients, Bone, doi:10.1016/j.bone.2021.116175
Ganmaa, Munkhzul, Fawzi, Spiegelman, Willett et al., High-Dose Vitamin D(3) during Tuberculosis Treatment in Mongolia. A Randomized Controlled Trial, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201705-0936OC
Gao, Ding, Dong, Zhang, Kursat Azkur et al., Risk factors for severe and critically ill COVID-19 patients: A review, Allergy, doi:10.1111/all.14657
Gao, Xie, Li, Liu, Yang, High Dose Vitamin D3 Supplementation Is Not Associated With Lower Mortality in Critically Ill Patients: A Meta-Analysis of Randomized Control Trials, Front. Nutr
Gregoriano, Koch, Haubitz, Conen, Fux et al., Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: An observational analysis, Swiss Med. Wkly, doi:10.4414/smw.2020.20316
Greiller, Martineau, Modulation of the immune response to respiratory viruses by vitamin D, Nutrients, doi:10.3390/nu7064240
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Hall, Cash, What is the real function of the liver 'function'tests?, Ulster Med. J
Hansdottir, Monick, Hinde, Lovan, Look et al., Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense, J. Immunol, doi:10.4049/jimmunol.181.10.7090
Hiremath, Cettomai, Baynes, Ratchford, Newsome et al., Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis, Mult. Scler. J, doi:10.1177/1352458509102844
Jaun, Boesing, Lüthi-Corridori, Abig, Makhdoomi et al., High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebocontrolled, multi-center study-VitCov Trial, Trials, doi:10.1186/s13063-022-06016-2
Jolliffe, Griffiths, Martineau, Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2012.11.017
Jordan, Siuka, Rotovnik, Pfeifer, COVID-19 and Vitamin D-a Systematic Review, Zdr. Varst, doi:10.2478/sjph-2022-0017
Karonova, Andreeva, Golovatuk, Bykova, Simanenkova et al., OH)D Level Is Associated with Severe Course and Poor Prognosis in COVID-19, Nutrients, doi:10.3390/nu13093021
Karonova, Golovatyuk, Kudryavtsev, Chernikova, Mikhaylova et al., Effect of Cholecalciferol Supplementation on the Clinical Features and Inflammatory Markers in Hospitalized COVID-19 Patients: A Randomized, Open-Label, Single-Center Study, Nutrients, doi:10.3390/nu14132602
Lin, Huang, Ju, Weng, Lee et al., Health-Related Quality of Life Measured by EQ-5D in Relation to Hospital Stay and Readmission in Elderly Patients Hospitalized for Acute Illness, Int. J. Environ. Res. Public. Health, doi:10.3390/ijerph17155333
Luan, Yin, Yao, Update Advances on C-Reactive Protein in COVID-19 and Other Viral Infections, Front. Immunol
Luo, Ballester, Soffientini, Jalan, Mehta, SARS-CoV-2 infection and liver involvement, Hepatol. Int, doi:10.1007/s12072-022-10364-1
Maghbooli, Sahraian, Jamalimoghadamsiahkali, Asadi, Zarei et al., Treatment with 25-Hydroxyvitamin D(3) (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial, Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol, doi:10.1016/j.eprac.2021.09.016
Malihi, Lawes, Wu, Huang, Waayer et al., Monthly highdose vitamin D supplementation does not increase kidney stone risk or serum calcium: Results from a randomized controlled trial, Am. J. Clin. Nutr, doi:10.1093/ajcn/nqy378
Malinverni, Ochogavia, Lecrenier, Scorpinniti, Preiser et al., Severe vitamin D deficiency in patients admitted to the emergency department with severe sepsis is associated with an increased 90-day mortality, Emerg. Med. J, doi:10.1136/emermed-2021-211973
Marando, Fusi-Schmidhauser, Tamburello, Grazioli, Gauthier et al., 1-year radiological, functional and quality-of-life outcomes in patients with SARS-CoV-2 pneumonia-A prospective observational study, NPJ Prim. Care Respir. Med, doi:10.1038/s41533-022-00273-z
Mariani, Antonietti, Tajer, Ferder, Inserra et al., High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial, PLoS ONE, doi:10.1371/journal.pone.0267918
Martineau, Maclaughlin, Hooper, Barnes, Jolliffe et al., Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs), Thorax, doi:10.1136/thoraxjnl-2014-206449
Mayadas, Cullere, Lowell, The Multifaceted Functions of Neutrophils, Annu. Rev. Pathol. Mech. Dis, doi:10.1146/annurev-pathol-020712-164023
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Noh, Kang, Kim, Syncope after Influenza Virus Infection, J. Korean Med. Sci
Olliver, Spelmink, Hiew, Meyer-Hoffert, Henriques-Normark et al., Immunomodulatory Effects of Vitamin D on Innate and Adaptive Immune Responses to Streptococcus pneumoniae, J. Infect. Dis, doi:10.1093/infdis/jit355
Park, Lee, Hong, Lim, Koh et al., Effect of vitamin D deficiency in Korean patients with acute respiratory distress syndrome, Korean J. Intern. Med, doi:10.3904/kjim.2017.380
Parohan, Yaghoubi, Seraji, Javanbakht, Sarraf et al., Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies, Aging Male, doi:10.1080/13685538.2020.1774748
Ramasamy, Vitamin D Metabolism and Guidelines for Vitamin D Supplementation, Clin. Biochem. Rev, doi:10.33176/AACB-20-00006
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study), Postgrad. Med. J, doi:10.1136/postgradmedj-2020-139065
Rosales, Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types?, Front. Physiol
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial, Nutrients, doi:10.3390/nu13072170
Saponaro, Franzini, Okoye, Antognoli, Campi et al., Is There a Crucial Link Between Vitamin D Status and Inflammatory Response in Patients with COVID-19? Front, Immunol
Sulli, Gotelli, Casabella, Paolino, Pizzorni et al., Vitamin D and Lung Outcomes in Elderly COVID-19 Patients, Nutrients, doi:10.3390/nu13030717
Tahir, Zahra, Neutrophilia, None
Tazerji, Shahabinejad, Tokasi, Rad, Khan et al., Global data analysis and risk factors associated with morbidity and mortality of COVID-19, Gene Rep, doi:10.1016/j.genrep.2022.101505
Torres, Casado, Vigón, Rodríguez-Mora, Mateos et al., Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.112965
Vijayakumar, Viswanathan, Aghoram, Idiopathic CD4 lymphocytopenia: Current insights, Immunotargets Ther
Wilk-Sledziewska, Sielatycki, Uscinska, Bujno, Rosolowski et al., The Impact of Cardiovascular Risk Factors on the Course of COVID-19, J. Clin. Med, doi:10.3390/jcm11082250
Xiang, Ma, Yu, Wang, Yin, Modeling the Global Dynamic Contagion of COVID-19, Front. Public Health, doi:10.3389/fpubh.2021.809987
Zhou, Yang, Zhou, Chen, Fang et al., A Review of SARS-CoV2: Compared With SARS-CoV and MERS-CoV, Front. Med, doi:10.3389/fmed.2021.628370