A Randomized Pilot Study Using Calcitriol in Hospitalized Patients
et al., Bone, doi:10.1016/j.bone.2021.116175, Sep 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
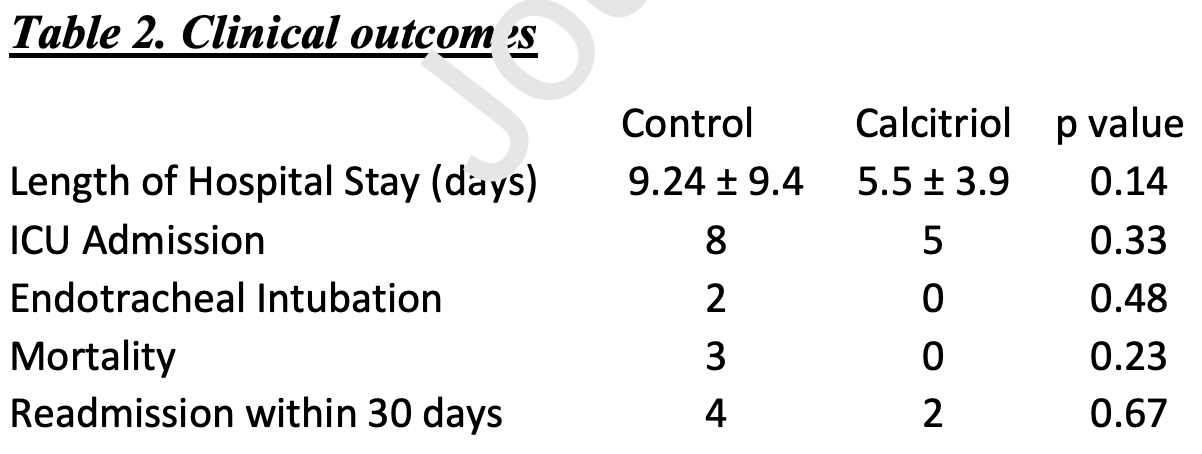
RCT 50 hospitalized patients in the USA, 25 treated with calcitriol, showing significantly improved oxygenation with treatment. Mortality, intubation, ICU admission, and hospitalization time also favored treatment, while not reaching statistical significance with the very small sample size.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 7th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 54th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 85.7% lower, RR 0.14, p = 0.23, treatment 0 of 25 (0.0%), control 3 of 25 (12.0%), NNT 8.3, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 80.0% lower, RR 0.20, p = 0.48, treatment 0 of 25 (0.0%), control 2 of 25 (8.0%), NNT 12, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 37.5% lower, RR 0.62, p = 0.33, treatment 5 of 25 (20.0%), control 8 of 25 (32.0%), NNT 8.3.
|
|
hospitalization time, 40.5% lower, relative time 0.60, p = 0.14, treatment 25, control 25.
|
|
relative Δ SaO2/FiO2, RR 0.14, p = 0.03, treatment 25, control 25, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Elamir et al., 8 Sep 2021, Randomized Controlled Trial, USA, peer-reviewed, 9 authors, study period September 2020 - December 2020, dosage calcitriol 0.5μg days 1-14.
A randomized pilot study using calcitriol in hospitalized COVID-19 patients
Bone, doi:10.1016/j.bone.2021.116175
Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company's public news and information website. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre -including this research content -immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active.
ACKNOWLEDGEMENTS There are no acknowledgements. that were also given losartan suggests that vitamin D functions, in part, by up-regulating the pulmonary renin/angiotensin system to mitigate lung damage. (19) This same system is disrupted by COVID-19 infection. (20) Human trials with cholecalciferol therapy for ARDS have mixed results. In a randomized trial, patients that underwent esophagectomy demonstrated a transient reduction in markers of ARDS with 300,000 IU treatment of cholecalciferol compared to placebo. (21) The authors argue that declining levels of VDBP that were observed in this trial may explain the short-lived benefit of cholecalciferol that was observed. In another randomized trial of 475 mechanically ventilated ICU patients given 540,000 units cholecalciferol vs placebo, a 44% reduction in hospital mortality was observed in the cholecalciferol treated patients among the subgroup of patients with 25-hydroxyvitamin D levels less than 12 ng/ml. (22) In addition, the cholecalciferol treated patients demonstrated a further reduction in mortality that persisted for 6 months of follow up. ( 22 ) A larger, multicenter trial is currently underway. (23) Limitations of our trial include lack of a placebo and lack of blinding. It is unlikely this would affect the SaO2/FIO2 endpoint. Additionally, 25-hydroxyvitamin D levels were not measured in our study. Enrollment of patients without regard to vitamin D status strengthens the generalizability of the results...
References
Amrein, Parekh, Westphal, Preiser, Berghold et al., Effect of highdose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study), BMJ Open
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of highdose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial, JAMA
Bilan, Dastranji, Behbahani, Comparison of the spo2/fio2 ratio and the pao2/fio2 ratio in patients with acute lung injury or acute respiratory distress syndrome, J Cardiovasc Thorac Res
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19, Eur J Endocrinol
Castillo, Costa, Barrios, Diaz, Lopez, None
Catoire, Tellier, De La Riviere, Beauvieux, Valdenaire et al., Assessment of the SpO2/FiO2 ratio as a tool for hypoxemia screening in the emergency department, Am J Emerg Med
Dancer, Parekh, Lax, Souza, Zheng et al., Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS), Thorax
Grein, Ohmagari, Shin, Diaz, Asperges et al., Compassionate Use of Remdesivir for Patients with Severe Covid-19, N Engl J Med
Guan, Zhong, Clinical Characteristics of Covid-19 in China. Reply, N Engl J Med
Henderson, Fink, Bassyouni, Argiropoulos, Brown et al., Vitamin D-Binding Protein Deficiency and Homozygous Deletion of the GC Gene, N Engl J Med
Hewison, Freeman, Hughes, Evans, Bland et al., Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells, J Immunol
Kong, Zhu, Shi, Liu, Chen et al., VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system, Mol Endocrinol
Laird, Rhodes, Kenny, Vitamin D and Inflammation: Potential Implications for Severity of Covid-19, Ir Med J
Lee, Eisman, Center, Vitamin D deficiency in critically ill patients, N Engl J Med
Miranda, Bouillon, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA
Nierman, Mechanick, Bone hyperresorption is prevalent in chronically critically ill patients, Chest
Parekh, Dancer, Scott, Souza, Howells et al., Vitamin D to Prevent Lung Injury Following Esophagectomy-A Randomized, Placebo-Controlled Trial, Crit Care Med
Rhodes, Dunstan, Laird, Subramanian, Kenny, COVID-19 mortality increases with northerly latitude after adjustment for age suggesting a link with ultraviolet and vitamin D, BMJ Nutr Prev Health
Santana, De Sousa, Amorim, Menezes, Araújo et al., SaO2/FiO2 ratio as risk stratification for patients with sepsis, Critical Care
Vaduganathan, Vardeny, Michel, Mcmurray, Pfeffer et al., None
Van Den Berghe, Van Roosbroeck, Vanhove, Wouters, Pourcq et al., Bone turnover in prolonged critical illness: effect of vitamin D, J Clin Endocrinol Metab
Waldron, Ashby, Cornes, Bechervaise, Razavi et al., Vitamin D: a negative acute phase reactant, J Clin Pathol
Zehnder, Bland, Williams, Mcninch, Howie et al., Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase, J Clin Endocrinol Metab
DOI record:
{
"DOI": "10.1016/j.bone.2021.116175",
"ISSN": [
"8756-3282"
],
"URL": "http://dx.doi.org/10.1016/j.bone.2021.116175",
"alternative-id": [
"S8756328221003410"
],
"article-number": "116175",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A randomized pilot study using calcitriol in hospitalized COVID-19 patients"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Bone"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.bone.2021.116175"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Inc. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Elamir",
"given": "Yasmine M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Amir",
"given": "Hajira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rana",
"given": "Yesha Patel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez",
"given": "Carolina Gonzalez",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feliciano",
"given": "Natalia Viera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omar",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grist",
"given": "William Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Via",
"given": "Michael A.",
"sequence": "additional"
}
],
"container-title": "Bone",
"container-title-short": "Bone",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
8
]
],
"date-time": "2021-09-08T22:46:43Z",
"timestamp": 1631141203000
},
"deposited": {
"date-parts": [
[
2023,
12,
26
]
],
"date-time": "2023-12-26T08:18:23Z",
"timestamp": 1703578703000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T21:11:57Z",
"timestamp": 1712610717796
},
"is-referenced-by-count": 40,
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S8756328221003410?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S8756328221003410?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "116175",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Clinical characteristics of Covid-19 in China. Reply",
"author": "Guan",
"first-page": "1861",
"issue": "19",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bone.2021.116175_bb0005",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007016",
"article-title": "Compassionate use of remdesivir for patients with severe Covid-19",
"author": "Grein",
"doi-asserted-by": "crossref",
"first-page": "2327",
"issue": "24",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bone.2021.116175_bb0010",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1530/EJE-20-0665",
"article-title": "Mechanisms in endocrinology: vitamin D and COVID-19",
"author": "Bilezikian",
"doi-asserted-by": "crossref",
"first-page": "R133",
"issue": "5",
"journal-title": "Eur. J. Endocrinol.",
"key": "10.1016/j.bone.2021.116175_bb0015",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.170.11.5382",
"article-title": "Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells",
"author": "Hewison",
"doi-asserted-by": "crossref",
"first-page": "5382",
"issue": "11",
"journal-title": "J. Immunol.",
"key": "10.1016/j.bone.2021.116175_bb0020",
"volume": "170",
"year": "2003"
},
{
"DOI": "10.1378/chest.114.4.1122",
"article-title": "Bone hyperresorption is prevalent in chronically critically ill patients",
"author": "Nierman",
"doi-asserted-by": "crossref",
"first-page": "1122",
"issue": "4",
"journal-title": "Chest.",
"key": "10.1016/j.bone.2021.116175_bb0025",
"volume": "114",
"year": "1998"
},
{
"DOI": "10.1210/jc.2003-030358",
"article-title": "Bone turnover in prolonged critical illness: effect of vitamin D",
"author": "Van den Berghe",
"doi-asserted-by": "crossref",
"first-page": "4623",
"issue": "10",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "10.1016/j.bone.2021.116175_bb0030",
"volume": "88",
"year": "2003"
},
{
"DOI": "10.1056/NEJMc0809996",
"article-title": "Vitamin D deficiency in critically ill patients",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "1912",
"issue": "18",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bone.2021.116175_bb0035",
"volume": "360",
"year": "2009"
},
{
"article-title": "Vitamin D and inflammation: potential implications for severity of Covid-19",
"author": "Laird",
"first-page": "81",
"issue": "5",
"journal-title": "Ir. Med. J.",
"key": "10.1016/j.bone.2021.116175_bb0040",
"volume": "113",
"year": "2020"
},
{
"DOI": "10.1136/bmjnph-2020-000110",
"article-title": "COVID-19 mortality increases with northerly latitude after adjustment for age suggesting a link with ultraviolet and vitamin D",
"author": "Rhodes",
"doi-asserted-by": "crossref",
"first-page": "118",
"issue": "1",
"journal-title": "BMJ Nutr. Prev. Health",
"key": "10.1016/j.bone.2021.116175_bb0045",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2021.01.092",
"article-title": "Assessment of the SpO2/FiO2 ratio as a tool for hypoxemia screening in the emergency department",
"author": "Catoire",
"doi-asserted-by": "crossref",
"first-page": "116",
"journal-title": "Am. J. Emerg. Med.",
"key": "10.1016/j.bone.2021.116175_bb0050",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1186/cc12951",
"article-title": "SaO2/FiO2 ratio as risk stratification for patients with sepsis",
"author": "Santana",
"doi-asserted-by": "crossref",
"first-page": "P51",
"issue": "4",
"journal-title": "Crit. Care",
"key": "10.1016/j.bone.2021.116175_bb0055",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.15171/jcvtr.2014.06",
"article-title": "Comparison of the spo2/fio2 ratio and the pao2/fio2 ratio in patients with acute lung injury or acute respiratory distress syndrome",
"author": "Bilan",
"doi-asserted-by": "crossref",
"first-page": "28",
"issue": "1",
"journal-title": "J. Cardiovasc. Thorac. Res.",
"key": "10.1016/j.bone.2021.116175_bb0060",
"volume": "7",
"year": "2015"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "crossref",
"first-page": "105751",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "10.1016/j.bone.2021.116175_bb0065",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial",
"author": "Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"issue": "11",
"journal-title": "JAMA.",
"key": "10.1016/j.bone.2021.116175_bb0070",
"volume": "325",
"year": "2021"
},
{
"article-title": "Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase",
"author": "Zehnder",
"first-page": "888",
"issue": "2",
"journal-title": "J. Clin. Endocrinol. Metab.",
"key": "10.1016/j.bone.2021.116175_bb0075",
"volume": "86",
"year": "2001"
},
{
"DOI": "10.1136/jclinpath-2012-201301",
"article-title": "Vitamin D: a negative acute phase reactant",
"author": "Waldron",
"doi-asserted-by": "crossref",
"first-page": "620",
"issue": "7",
"journal-title": "J. Clin. Pathol.",
"key": "10.1016/j.bone.2021.116175_bb0080",
"volume": "66",
"year": "2013"
},
{
"DOI": "10.1056/NEJMoa1807841",
"article-title": "Vitamin D-binding protein deficiency and homozygous deletion of the GC gene",
"author": "Henderson",
"doi-asserted-by": "crossref",
"first-page": "1150",
"issue": "12",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bone.2021.116175_bb0085",
"volume": "380",
"year": "2019"
},
{
"DOI": "10.1136/thoraxjnl-2014-206680",
"article-title": "Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS)",
"author": "Dancer",
"doi-asserted-by": "crossref",
"first-page": "617",
"issue": "7",
"journal-title": "Thorax.",
"key": "10.1016/j.bone.2021.116175_bb0090",
"volume": "70",
"year": "2015"
},
{
"DOI": "10.1210/me.2013-1146",
"article-title": "VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system",
"author": "Kong",
"doi-asserted-by": "crossref",
"first-page": "2116",
"issue": "12",
"journal-title": "Mol. Endocrinol.",
"key": "10.1016/j.bone.2021.116175_bb0095",
"volume": "27",
"year": "2013"
},
{
"DOI": "10.1056/NEJMsr2005760",
"article-title": "Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19",
"author": "Vaduganathan",
"doi-asserted-by": "crossref",
"first-page": "1653",
"issue": "17",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.bone.2021.116175_bb0100",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000003405",
"article-title": "Vitamin D to prevent lung injury following esophagectomy—a randomized, placebo-controlled trial",
"author": "Parekh",
"doi-asserted-by": "crossref",
"first-page": "e1128",
"issue": "12",
"journal-title": "Crit. Care Med.",
"key": "10.1016/j.bone.2021.116175_bb0105",
"volume": "46",
"year": "2018"
},
{
"DOI": "10.1001/jama.2014.13204",
"article-title": "Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial",
"author": "Amrein",
"doi-asserted-by": "crossref",
"first-page": "1520",
"issue": "15",
"journal-title": "JAMA.",
"key": "10.1016/j.bone.2021.116175_bb0110",
"volume": "312",
"year": "2014"
},
{
"DOI": "10.1136/bmjopen-2019-031083",
"article-title": "Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study)",
"author": "Amrein",
"doi-asserted-by": "crossref",
"first-page": "e031083",
"issue": "11",
"journal-title": "BMJ Open",
"key": "10.1016/j.bone.2021.116175_bb0115",
"volume": "9",
"year": "2019"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S8756328221003410"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Histology",
"Physiology",
"Endocrinology, Diabetes and Metabolism"
],
"subtitle": [],
"title": "A randomized pilot study using calcitriol in hospitalized COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "154"
}
