A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D — a randomised multicentre international clinical trial
et al., BMC Medicine, doi:10.1186/s12916-022-02290-8, COVID-VIT-D, NCT04552951, Feb 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
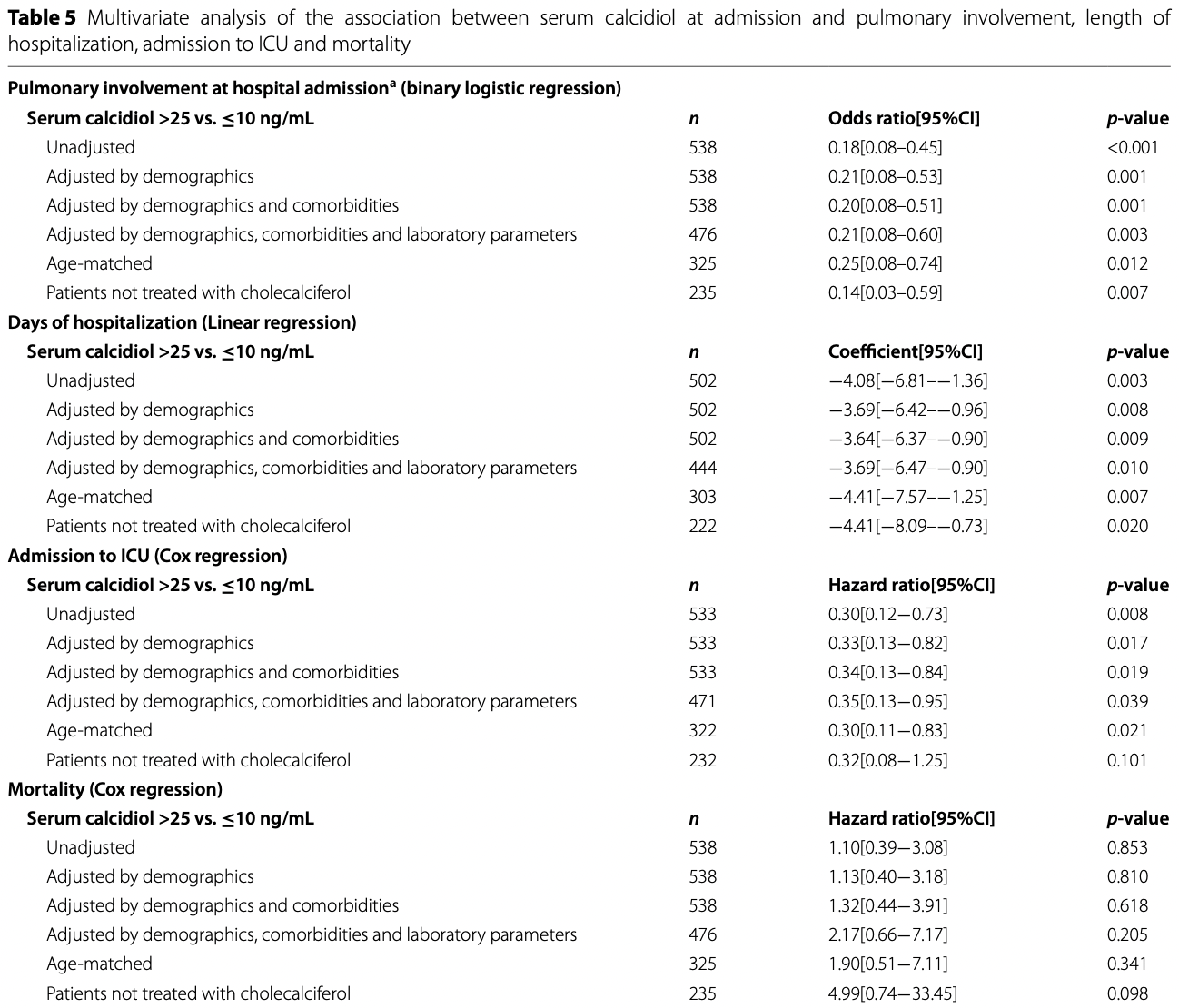
RCT 274 very late stage (>80% pulmonary involvement at baseline) hospitalized COVID-19 patients treated with a single dose of cholecalciferol, and 269 control patients, showing no significant differences. High serum calcidiol levels at admission were associated with lower pulmonary involvement, shorter hospitalization, and lower ICU admission.
Serum levels increased in the treatment group, however average levels were still insufficient at discharge. Calcifediol or calcitriol, which avoids several days delay in conversion, may be more successful, especially with this very late stage usage.
100,000IU cholecalciferol.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 13th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 72nd of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
This study is excluded in the after exclusion results of meta-analysis:
very late stage study using cholecalciferol instead of calcifediol or calcitriol.
|
risk of death, 44.0% higher, RR 1.44, p = 0.31, treatment 22 of 274 (8.0%), control 15 of 269 (5.6%).
|
|
risk of ICU admission, 4.9% higher, RR 1.05, p = 0.82, treatment 47 of 274 (17.2%), control 44 of 269 (16.4%).
|
|
hospitalization time, 5.3% higher, relative time 1.05, treatment 274, control 269.
|
|
risk of death, 117.0% higher, RR 2.17, p = 0.20, high D levels 87, low D levels 96, >25 vs. ≤10 ng/mL, adjusted by demographics, comorbidities, and laboratory parameters, outcome based on serum levels.
|
|
risk of ICU admission, 65.0% lower, RR 0.35, p = 0.04, high D levels 87, low D levels 96, >25 vs. ≤10 ng/mL, adjusted by demographics, comorbidities, and laboratory parameters, outcome based on serum levels.
|
|
risk of progression, 79.0% lower, RR 0.21, p = 0.003, high D levels 87, low D levels 96, pulmonary involvment at admission, >25 vs. ≤10 ng/mL, adjusted by demographics, comorbidities, and laboratory parameters, outcome based on serum levels.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cannata-Andía et al., 18 Feb 2022, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 59.0, 22 authors, study period 4 April, 2020 - 22 April, 2021, dosage 100,000IU single dose, trial NCT04552951 (history) (COVID-VIT-D).
A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D—a randomised multicentre international clinical trial
BMC Medicine, doi:10.1186/s12916-022-02290-8
Background: Vitamin D status has been implicated in COVID-19 disease. The objective of the COVID-VIT-D trial was to investigate if an oral bolus of cholecalciferol (100,000 IU) administered at hospital admission influences the outcomes of moderate-severe COVID-19 disease. In the same cohort, the association between baseline serum calcidiol levels with the same outcomes was also analysed.
Methods: The COVID-VIT-D is a multicentre, international, randomised, open label, clinical trial conducted throughout 1 year. Patients older than 18 years with moderate-severe COVID-19 disease requiring hospitalisation were included. At admission, patients were randomised 1:1 to receive a single oral bolus of cholecalciferol (n=274) or nothing (n=269). Patients were followed from admission to discharge or death. Length of hospitalisation, admission to intensive care unit (ICU) and mortality were assessed. Results: In the randomised trial, comorbidities, biomarkers, symptoms and drugs used did not differ between groups. Median serum calcidiol in the cholecalciferol and control groups were 17.0 vs. 16.1 ng/mL at admission and 29.0 vs. 16.4 ng/mL at discharge, respectively. The median length of hospitalisation (10.
Abbreviations AEMPs: Spanish Agency for Medicines and Health Products; BMI: Body mass index; CAT : Computed axial tomography; CRP: C-reactive protein; HUCA : Hospital Universitario Central de Asturias; ICU: Intensive care unit; IL-6: Interleukin 6; PCR: Polymerase chain reaction; SARS-CoV-2: Severe Acute Respiratory Syndrome CoronaVirus 2.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12916-022-02290-8. S1 . Variables collected in the COVID-VIT-D trial. T. Table S2 . Symptoms at discharge. Table S3 . Biochemical parameters at discharge. Table S4 . Demographic, comorbidities, and serum calcidiol categories at hospital admission. Table S5 . Relevant biochemical parameters and serum calcidiol categories at hospital admission. Table S6 . Relevant biochemical parameters and serum calcidiol categories at hospital admission in age-matched patients. Table S7 . Pulmonary involvement at admission and outcomes according to serum calcidiol categories. Table S8 . Types and number of drugs received during the hospitalization and serum calcidiol categories at hospital admission. Table S9 . Demographic, comorbidities, and serum calcidiol categories at admission in agematched patients. Table S10 . Types and number of drugs received during the hospitalization and serum calcidiol categories at hospital admission in age-matched patients. Table S11 . Relevant biochemical parameters and serum calcidiol..
References
Alvarez, Aguilar-Jimenez, Rugeles, The potential protective role of vitamin D supplementation on HIV-1 infection, Front Immunol
Alvarez-Hernández, Gómez-Alonso, Jb, Vitamin D supplementation: what is right?, Clin Cases Miner Bone Metab
Amrein, Parekh, Westphal, Preiser, Berghold et al., Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study), BMJ open
Asensio, Mauricio, Huca, None
Ayelign, Workneh, Molla, Role of vitamin-D supplementation in TB/HIV co-infected patients, Infect Drug Resist
Bjorkhem-Bergman, Missailidis, Karlsson-Valik, Tammelin, Ekstrom et al., Vitamin D supplementation to persistent carriers of MRSA-a randomized and placebo-controlled clinical trial, Eur J Clin Microbiol Infect Dis
Camargo, Sluyter, Stewart, Khaw, Lawes et al., Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial, Clin Infect Dis
Cannata-Andia, Gomez, Vitamin D deficiency: a neglected aspect of disturbed calcium metabolism in renal failure, Nephrol Dial Transplant
Castillo, Costa, Barrios, Diaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study, J Steroid Biochem Mol Biol
Chowdhury, Kunutsor, Vitezova, Oliver-Williams, Chowdhury et al., Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies, BMJ (Clinical research ed
Christakos, Dhawan, Verstuyf, Verlinden, Carmeliet, Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects, Physiol Rev
Coussens, Martineau, Wilkinson, Anti-inflammatory and antimicrobial actions of vitamin d in combating TB/HIV, Scientifica
Dusso, Molecular biology of Vitamin D: genomic and nongenomic actions of vitamin D in chronic kidney disease
Fernández-Martín, Canteros, Alles, Massari, Cannata-Andía, Aluminum exposure in chronic renal failure in iberoamerica at the end of the 1990s: overview and perspectives, Am J Med Sci
Ferrari, Daniela, Hblt, Chile, None
Gaksch, Jorde, Grimnes, Joakimsen, Schirmer et al., Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium, PLoS One
Ganmaa, Enkhmaa, Nasantogtokh, Sukhbaatar, Tumur-Ochir et al., Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials, J Intern Med
Ganmaa, Uyanga, Zhou, Gantsetseg, Delgerekh et al., Vitamin D supplements for prevention of tuberculosis infection and disease, New Engl J Med
Gassen, Niemeyer, Muth, Corman, Martinelli et al., SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection, Nat Commun
Ginde, Blatchford, Breese, Zarrabi, Linnebur et al., High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial, J Am Geriatr Soc
Ginde, Brower, Caterino, Finck, Banner-Goodspeed et al., Early high-dose vitamin D(3) for critically ill, vitamin D-deficient patients, N Engl J Med
Grandi, Breitling, Vossen, Hahmann, Wüsten et al., Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease, Am Heart J
Grant, Review of recent advances in understanding the role of vitamin D in reducing cancer risk: breast, colorectal, prostate, and overall cancer, Anticancer Res
Haykal, Samji, Zayed, Gakhal, Dhillon et al., The role of vitamin D supplementation for primary prevention of cancer: metaanalysis of randomized controlled trials, J Community Hosp Intern Med Perspect
Heart, Institute, Ginde, Brower, Caterino et al., Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients, New Engl J Med
Hernandez, Nan, Fernandez-Ayala, Garcia-Unzueta, Hernandez-Hernandez et al., Vitamin D status in hospitalized patients with SARS-CoV-2 infection, J Clin Endocrinol Metab
Holick, Vitamin, Physiology, molecular biology, and clinical applications
Hueniken, Aglipay, Birken, Parkin, Loeb et al., Effect of high-dose vitamin D supplementation on upper respiratory tract infection symptom severity in healthy children, Pediatr Infect Dis J
Illescas-Montes, Melguizo-Rodríguez, Ruiz, Vj, Vitamin D and autoimmune diseases, Life Sci
Jetty, Glueck, Wang, Shah, Prince et al., Safety of 50,000-100,000 units of vitamin D3/week in vitamin D-deficient, hypercholesterolemic patients with reversible statin intolerance, North Am J Med Sci
Jimenez-Sousa, Martinez, Medrano, Fernandez-Rodriguez, Resino, Vitamin D in human immunodeficiency virus infection: influence on immunity and disease, Front Immunol
Johansson, Odén, Kanis, Mccloskey, Lorentzon et al., Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden, Osteoporos Int
Jolliffe, Camargo, Jr, Sluyter, Aglipay et al., Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol
Kasahara, Singh, Noymer, Vitamin D (25OHD) serum seasonality in the United States, PLoS One
Kearns, Alvarez, Tangpricha, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract
Liu, Fang, Wu, Tan, Zhou et al., 1,25-(OH)(2)D(3)/Vitamin D receptor alleviates systemic lupus erythematosus by downregulating Skp2 and upregulating p27, Cell Commun Signal
Lopez, Jorgetti, Caorsi, Ferreira, Palma et al., Epidemiology of renal osteodystrophy in Iberoamerica, Nephrol Dial Transplant
López, Jb, El amplio espectro de la activación del receptor de vitamina D
Malihi, Wu, Lawes, Scragg, Adverse events from large dose vitamin D supplementation taken for one year or longer, J Steroid Biochem Mol Biol
Manson, Cook, Lee, Bassuk, Mora, Vitamin D supplements and prevention of cancer and cardiovascular disease, New Engl J Med
Mansueto, Seidita, Vitale, Gangemi, Iaria et al., Vitamin D deficiency in HIV infection: not only a bone disorder, BioMed Res Int
Manzano, Bierzo, None
Martínez-Alonso, Dusso, Ariza, Nabal, Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: a cross-sectional study, Palliat Med
Mazess, Bischoff-Ferrari, Hughes, Vitamin D: bolus is bogus-a narrative review, JBMR Plus
Melamed, Michos, Post, Astor, 25-hydroxyvitamin D levels and the risk of mortality in the general population, Arch Intern Med
Merker, Amsler, Pereira, Bolliger, Tribolet et al., Vitamin D deficiency is highly prevalent in malnourished inpatients and associated with higher mortality: a prospective cohort study, Medicine
Metzger, Stengel, Epidemiology of vitamin D deficiency in chronic kidney disease
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial, Jama
Naves-Diaz, Alvarez-Hernandez, Passlick-Deetjen, Guinsburg, Marelli et al., Oral active vitamin D is associated with improved survival in hemodialysis patients, Kidney Int
Naves-Diaz, Passlick-Deetjen, Guinsburg, Marelli, Fernandez-Martin et al., Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study, Nephrol Dial Transplant
Naves-Díaz, Cabezas-Rodríguez, Barrio-Vázquez, Fernández, Díaz-López et al., Low calcidiol levels and risk of progression of aortic calcification, Osteoporos Int
Nogues, Ovejero, Pineda-Moncusí, Bouillon, Arenas et al., Calcifediol treatment and COVID-19-related outcomes, J Clin Endocrinol Metab
Oddo, Hospital Independencia
Onetto, None
Ortega, The cholecalciferol was friendly donated to HUCA by Gebro Pharma from April to
Paganti, Luciana, Hmc, None
Pal, Banerjee, Bhadada, Shetty, Singh et al., Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis, J Endocrinol Invest
Perge, Boros, Gellér, Osztheimer, Szilágyi et al., Vitamin D deficiency predicts poor clinical outcomes in heart failure patients undergoing cardiac resynchronization therapy, Dis Markers
Pinzone, Rosa, Malaguarnera, Madeddu, Focà et al., Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic, Eur Rev Med Pharmacol Sci
Rodríguez, María, Bierzo, None
Sassi, Tamone, Amelio, Vitamin D: nutrient, hormone, and immunomodulator, Nutrients
Scragg, Stewart, Waayer, Lawes, Toop et al., Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial, JAMA Cardiol
Sudfeld, Mugusi, Aboud, Nagu, Wang et al., Efficacy of vitamin D(3) supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial, Trials
Sudfeld, Mugusi, Muhihi, Aboud, Nagu et al., Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebocontrolled trial, Lancet HIV
Tarracina, Laura, Hmc, None
Teresa, Huca, None
Terán, María, Hospital, Bierzo, None
Tukvadze, Sanikidze, Kipiani, Hebbar, Easley et al., High-dose vitamin D3 in adults with pulmonary tuberculosis: a doubleblind randomized controlled trial, Am J Clin Nutr
Vazquez, Huerta-Delgado, Castillo, Villarreal-Calderón, Gonzalez-Gil et al., Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study, Int J Mol Sci
Vieth, Chapter 57 -The pharmacology of vitamin D
Vine, Hospital Barros Luco Trudeau -HBLT-and Universidad de Chile -U Chile
Wejse, Gomes, Rabna, Gustafson, Aaby et al., Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial, Am J Respir Crit Care Med
Yang, Ou-Yang, Huang, Low serum vitamin D levels increase the mortality of cardiovascular disease in older adults: a dose-response meta-analysis of prospective studies, Medicine
Zhang, Chen, Yang, Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: a metaanalysis of randomized controlled trials, Chin Med J
Zhang, Fang, Tang, Jia, Feng et al., Association between vitamin D supplementation and mortality: systematic review and metaanalysis, Bmj
Zittermann, Ernst, Prokop, Fuchs, Dreier et al., Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily, Eur Heart J
Zittermann, Iodice, Pilz, Grant, Bagnardi et al., Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies, Am J Clin Nutr
DOI record:
{
"DOI": "10.1186/s12916-022-02290-8",
"ISSN": [
"1741-7015"
],
"URL": "http://dx.doi.org/10.1186/s12916-022-02290-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Vitamin D status has been implicated in COVID-19 disease. The objective of the COVID-VIT-D trial was to investigate if an oral bolus of cholecalciferol (100,000 IU) administered at hospital admission influences the outcomes of moderate-severe COVID-19 disease. In the same cohort, the association between baseline serum calcidiol levels with the same outcomes was also analysed.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The COVID-VIT-D is a multicentre, international, randomised, open label, clinical trial conducted throughout 1 year. Patients older than 18 years with moderate-severe COVID-19 disease requiring hospitalisation were included. At admission, patients were randomised 1:1 to receive a single oral bolus of cholecalciferol (<jats:italic>n</jats:italic>=274) or nothing (<jats:italic>n</jats:italic>=269). Patients were followed from admission to discharge or death. Length of hospitalisation, admission to intensive care unit (ICU) and mortality were assessed.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In the randomised trial, comorbidities, biomarkers, symptoms and drugs used did not differ between groups. Median serum calcidiol in the cholecalciferol and control groups were 17.0 vs<jats:italic>.</jats:italic> 16.1 ng/mL at admission and 29.0 vs<jats:italic>.</jats:italic> 16.4 ng/mL at discharge, respectively. The median length of hospitalisation (10.0 [95%CI 9.0–10.5] vs<jats:italic>.</jats:italic> 9.5 [95%CI 9.0–10.5] days), admission to ICU (17.2% [95%CI 13.0–22.3] vs. 16.4% [95%CI 12.3–21.4]) and death rate (8.0% [95%CI 5.2–12.1] vs<jats:italic>.</jats:italic> 5.6% [95%CI 3.3–9.2]) did not differ between the cholecalciferol and control group. In the cohort analyses, the highest serum calcidiol category at admission (>25ng/mL) was associated with lower percentage of pulmonary involvement and better outcomes.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The randomised clinical trial showed the administration of an oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve the outcomes of the COVID-19 disease. A cohort analysis showed that serum calcidiol at hospital admission was associated with outcomes.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>COVID-VIT-D trial was authorised by the Spanish Agency for Medicines and Health products (AEMPS) and registered in European Union Drug Regulating Authorities Clinical Trials (EudraCT 2020-002274-28) and in ClinicalTrials.gov (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04552951\">NCT04552951</jats:ext-link>).</jats:p>\n </jats:sec>",
"alternative-id": [
"2290"
],
"article-number": "83",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "30 August 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "9 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "18 February 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The ethics committees of all participating centre approved the study. Due to the COVID-19 pandemic and in order to avoid unnecessary exposure to the SARS-CoV-2 virus, all ethics committees authorized verbal consent. The trial was conducted according to the ethical principles of the Declaration of Helsinki."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The following authors received research grants fees, grants for congress attending, courses and collaborations by the following entities: Jorge B. Cannata-Andía from Amgen, Kyowa-Kirim and Vifor Pharma; Ricardo Mouzo from Takeda, Otsuka, Nipro, Sanofi-Aventis, Amgen and the Senefro Foundation; Natalia Carrillo-López from Ministerio de Ciencia e Innovación (MICINN)/Instituto de Salud Carlos III (ISCIII); Sara Panizo from MICINN/ISCIII and Luis Hernando gran from Fundación Renal Íñigo Álvarez de Toledo; Carolina Ballarino from Pfizer, Takeda and Sanofi-Aventis; Jacqueline Pefaur-Penna from Novartis and Sanofi-Aventis; Jesús Calviño-Varela from Baxter, Otsuka, Palex, Astra, Vifor and Chiesi; Carlos Gómez-Alonso from Amgen, UCB, Stada, Grünenthal, Gebro Pharma, FAES, Kiowa-Kirin and Laboratorios Rubió; John Cunningham from Amgen, Merck and Vifor Pharma; Manuel Naves-Díaz from MICINN/ISCIII, Amgen, UCB, Kyowa-Kirim, Stada, Italfármaco, Gebro Pharma, Rubió, Gedeon Richter, Grünenthal and FEIOMM and José L. Fernández-Martín from MICINN/ISCIII. The rest of authors are not aware of any additional relationship, funding or financial holdings."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6543-9960",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cannata-Andía",
"given": "Jorge B.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Díaz-Sottolano",
"given": "Augusto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández",
"given": "Pehuén",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palomo-Antequera",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herrero-Puente",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mouzo",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrillo-López",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Panizo",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibañez",
"given": "Guillermo H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cusumano",
"given": "Carlos A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ballarino",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Polo",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pefaur-Penna",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maderuelo-Riesco",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calviño-Varela",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez",
"given": "Mónica D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Alonso",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunningham",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naves-Díaz",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Douthat",
"given": "Walter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández-Martín",
"given": "José L.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the COVID-VIT-D trial collaborators",
"sequence": "additional"
}
],
"container-title": [
"BMC Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
18
]
],
"date-time": "2022-02-18T02:02:49Z",
"timestamp": 1645149769000
},
"deposited": {
"date-parts": [
[
2022,
2,
19
]
],
"date-time": "2022-02-19T00:25:22Z",
"timestamp": 1645230322000
},
"indexed": {
"date-parts": [
[
2022,
2,
19
]
],
"date-time": "2022-02-19T00:40:40Z",
"timestamp": 1645231240449
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1741-7015"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2022,
2,
18
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
18
]
],
"date-time": "2022-02-18T00:00:00Z",
"timestamp": 1645142400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
18
]
],
"date-time": "2022-02-18T00:00:00Z",
"timestamp": 1645142400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12916-022-02290-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12916-022-02290-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12916-022-02290-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
2,
18
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
18
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1007/978-1-60327-303-9",
"author": "MF Holick",
"doi-asserted-by": "crossref",
"key": "2290_CR1",
"unstructured": "Holick MF, Vitamin D. Physiology, molecular biology, and clinical applications: Totowa. NJ: Humana Press; 2010.",
"volume-title": "Physiology, molecular biology, and clinical applications: Totowa",
"year": "2010"
},
{
"DOI": "10.3390/nu10111656",
"author": "F Sassi",
"doi-asserted-by": "crossref",
"first-page": "1656",
"issue": "11",
"journal-title": "Nutrients.",
"key": "2290_CR2",
"unstructured": "Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656.",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1016/j.lfs.2019.116744",
"author": "R Illescas-Montes",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci.",
"key": "2290_CR3",
"unstructured": "Illescas-Montes R, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Vitamin D and autoimmune diseases. Life Sci. 2019;233:116744.",
"volume": "233",
"year": "2019"
},
{
"DOI": "10.1152/physrev.00014.2015",
"author": "S Christakos",
"doi-asserted-by": "crossref",
"first-page": "365",
"issue": "1",
"journal-title": "Physiol Rev.",
"key": "2290_CR4",
"unstructured": "Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408.",
"volume": "96",
"year": "2016"
},
{
"DOI": "10.1155/2014/903680",
"author": "AK Coussens",
"doi-asserted-by": "crossref",
"journal-title": "Scientifica.",
"key": "2290_CR5",
"unstructured": "Coussens AK, Martineau AR, Wilkinson RJ. Anti-inflammatory and antimicrobial actions of vitamin d in combating TB/HIV. Scientifica. 2014;2014:903680.",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.3389/fimmu.2019.02291",
"author": "N Alvarez",
"doi-asserted-by": "crossref",
"first-page": "2291",
"journal-title": "Front Immunol",
"key": "2290_CR6",
"unstructured": "Alvarez N, Aguilar-Jimenez W, Rugeles MT. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10:2291.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/S2352-3018(20)30108-9",
"author": "CR Sudfeld",
"doi-asserted-by": "crossref",
"first-page": "e463",
"issue": "7",
"journal-title": "Lancet HIV.",
"key": "2290_CR7",
"unstructured": "Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga N, et al. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463–e71.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1093/ndt/17.11.1875",
"author": "JB Cannata-Andia",
"doi-asserted-by": "crossref",
"first-page": "1875",
"issue": "11",
"journal-title": "Nephrol Dial Transplant.",
"key": "2290_CR8",
"unstructured": "Cannata-Andia JB, Gomez AC. Vitamin D deficiency: a neglected aspect of disturbed calcium metabolism in renal failure. Nephrol Dial Transplant. 2002;17(11):1875–8.",
"volume": "17",
"year": "2002"
},
{
"DOI": "10.1056/NEJMoa1911124",
"author": "L National Heart",
"doi-asserted-by": "crossref",
"first-page": "2529",
"issue": "26",
"journal-title": "New Engl J Med",
"key": "2290_CR9",
"unstructured": "National Heart L, Blood Institute PCTN, Ginde AA, Brower RG, Caterino JM, Finck L, et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. New Engl J Med. 2019;381(26):2529–40.",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1097/MD.0000000000018113",
"author": "M Merker",
"doi-asserted-by": "crossref",
"issue": "48",
"journal-title": "Medicine (Baltimore).",
"key": "2290_CR10",
"unstructured": "Merker M, Amsler A, Pereira R, Bolliger R, Tribolet P, Braun N, et al. Vitamin D deficiency is highly prevalent in malnourished inpatients and associated with higher mortality: a prospective cohort study. Medicine (Baltimore). 2019;98(48):e18113.",
"volume": "98",
"year": "2019"
},
{
"DOI": "10.1136/bmjopen-2019-031083",
"author": "K Amrein",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "BMJ open.",
"key": "2290_CR11",
"unstructured": "Amrein K, Parekh D, Westphal S, Preiser JC, Berghold A, Riedl R, et al. Effect of high-dose vitamin D3 on 28-day mortality in adult critically ill patients with severe vitamin D deficiency: a study protocol of a multicentre, placebo-controlled double-blind phase III RCT (the VITDALIZE study). BMJ open. 2019;9(11):e031083.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1007/s00198-011-1809-5",
"author": "H Johansson",
"doi-asserted-by": "crossref",
"first-page": "991",
"issue": "3",
"journal-title": "Osteoporos Int.",
"key": "2290_CR12",
"unstructured": "Johansson H, Odén A, Kanis J, McCloskey E, Lorentzon M, Ljunggren Ö, et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporos Int. 2012;23(3):991–9.",
"volume": "23",
"year": "2012"
},
{
"author": "J Yang",
"issue": "34",
"journal-title": "Medicine (Baltimore).",
"key": "2290_CR13",
"unstructured": "Yang J, Ou-Yang J, Huang J. Low serum vitamin D levels increase the mortality of cardiovascular disease in older adults: a dose-response meta-analysis of prospective studies. Medicine (Baltimore). 2019;98(34):e16733.",
"volume": "98",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2018.00458",
"author": "MA Jimenez-Sousa",
"doi-asserted-by": "crossref",
"first-page": "458",
"journal-title": "Front Immunol",
"key": "2290_CR14",
"unstructured": "Jimenez-Sousa MA, Martinez I, Medrano LM, Fernandez-Rodriguez A, Resino S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front Immunol. 2018;9:458.",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"author": "M Entrenas Castillo",
"doi-asserted-by": "crossref",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2290_CR15",
"unstructured": "Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcala Diaz JF, Lopez Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751.",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1210/clinem/dgaa733",
"author": "JL Hernandez",
"doi-asserted-by": "crossref",
"first-page": "e1343",
"issue": "3",
"journal-title": "J Clin Endocrinol Metab.",
"key": "2290_CR16",
"unstructured": "Hernandez JL, Nan D, Fernandez-Ayala M, Garcia-Unzueta M, Hernandez-Hernandez MA, Lopez-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e53.",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1007/s00198-011-1550-0",
"author": "M Naves-Díaz",
"doi-asserted-by": "crossref",
"first-page": "1177",
"issue": "3",
"journal-title": "Osteoporos Int.",
"key": "2290_CR17",
"unstructured": "Naves-Díaz M, Cabezas-Rodríguez I, Barrio-Vázquez S, Fernández E, Díaz-López JB, Cannata-Andía JB. Low calcidiol levels and risk of progression of aortic calcification. Osteoporos Int. 2012;23(3):1177–82.",
"volume": "23",
"year": "2012"
},
{
"DOI": "10.1093/eurheartj/ehx235",
"author": "A Zittermann",
"doi-asserted-by": "crossref",
"first-page": "2279",
"issue": "29",
"journal-title": "Eur Heart J",
"key": "2290_CR18",
"unstructured": "Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38(29):2279–86.",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.1002/jbm4.10567",
"author": "RB Mazess",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "JBMR Plus.",
"key": "2290_CR19",
"unstructured": "Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus-a narrative review. JBMR Plus. 2021;5(12):e10567.",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1210/clinem/dgab405",
"author": "X Nogues",
"doi-asserted-by": "crossref",
"first-page": "e4017",
"issue": "10",
"journal-title": "J Clin Endocrinol Metab.",
"key": "2290_CR20",
"unstructured": "Nogues X, Ovejero D, Pineda-Moncusí M, Bouillon R, Arenas D, Pascual J, et al. Calcifediol treatment and COVID-19-related outcomes. J Clin Endocrinol Metab. 2021;106(10):e4017–e27.",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1111/joim.13399",
"author": "D Ganmaa",
"doi-asserted-by": "crossref",
"first-page": "141",
"issue": "2",
"journal-title": "J Intern Med.",
"key": "2290_CR21",
"unstructured": "Ganmaa D, Enkhmaa D, Nasantogtokh E, Sukhbaatar S, Tumur-Ochir KE, Manson JE. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J Intern Med. 2022;291(2):141–64.",
"volume": "291",
"year": "2022"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"author": "DA Jolliffe",
"doi-asserted-by": "crossref",
"first-page": "276",
"issue": "5",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "2290_CR22",
"unstructured": "Jolliffe DA, Camargo CA Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–92.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.4158/EP13265.RA",
"author": "MD Kearns",
"doi-asserted-by": "crossref",
"first-page": "341",
"issue": "4",
"journal-title": "Endocr Pract",
"key": "2290_CR23",
"unstructured": "Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014;20(4):341–51.",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1016/j.jsbmb.2018.12.002",
"author": "Z Malihi",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2290_CR24",
"unstructured": "Malihi Z, Wu Z, Lawes CMM, Scragg R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J Steroid Biochem Mol Biol. 2019;188:29–37.",
"volume": "188",
"year": "2019"
},
{
"DOI": "10.1111/jgs.14679",
"author": "AA Ginde",
"doi-asserted-by": "crossref",
"first-page": "496",
"issue": "3",
"journal-title": "J Am Geriatr Soc.",
"key": "2290_CR25",
"unstructured": "Ginde AA, Blatchford P, Breese K, Zarrabi L, Linnebur SA, Wallace JI, et al. High-dose monthly vitamin D for prevention of acute respiratory infection in older long-term care residents: a randomized clinical trial. J Am Geriatr Soc. 2017;65(3):496–503.",
"volume": "65",
"year": "2017"
},
{
"DOI": "10.4103/1947-2714.179133",
"author": "V Jetty",
"doi-asserted-by": "crossref",
"first-page": "156",
"issue": "3",
"journal-title": "North Am J Med Sci",
"key": "2290_CR26",
"unstructured": "Jetty V, Glueck CJ, Wang P, Shah P, Prince M, Lee K, et al. Safety of 50,000-100,000 units of vitamin D3/week in vitamin D-deficient, hypercholesterolemic patients with reversible statin intolerance. North Am J Med Sci. 2016;8(3):156–62.",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1164/rccm.200804-567OC",
"author": "C Wejse",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "9",
"journal-title": "Am J Respir Crit Care Med",
"key": "2290_CR27",
"unstructured": "Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50.",
"volume": "179",
"year": "2009"
},
{
"DOI": "10.1016/B978-0-12-381978-9.10057-5",
"author": "R Vieth",
"doi-asserted-by": "crossref",
"edition": "3",
"first-page": "1041",
"key": "2290_CR28",
"unstructured": "Vieth R. Chapter 57 - The pharmacology of vitamin D. In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3rd ed. San Diego: Academic; 2011. p. 1041–66.",
"volume-title": "Vitamin D",
"year": "2011"
},
{
"author": "JB Díaz López",
"first-page": "275",
"key": "2290_CR29",
"unstructured": "Díaz López JB, Cannata-Andía JB. El amplio espectro de la activación del receptor de vitamina D. In: Cannata-Andía JB, editor. Alteraciones del metabolismo óseo y mineral en la enfermedad renal crónica: avances en patogenia, diagnóstico y tratamiento. Barcelona Wolters Kluwer: Lippincott Williams & Wilkins; 2010. p. 275–9.",
"volume-title": "Alteraciones del metabolismo óseo y mineral en la enfermedad renal crónica: avances en patogenia, diagnóstico y tratamiento",
"year": "2010"
},
{
"author": "D Alvarez-Hernández",
"first-page": "71",
"journal-title": "Clin Cases Miner Bone Metab",
"key": "2290_CR30",
"unstructured": "Alvarez-Hernández D, Gómez-Alonso C, Cannata-Andía JB. Vitamin D supplementation: what is right? Clin Cases Miner Bone Metab. 2006;3:71–5.",
"volume": "3",
"year": "2006"
},
{
"DOI": "10.21873/anticanres.13977",
"author": "WB Grant",
"doi-asserted-by": "crossref",
"first-page": "491",
"issue": "1",
"journal-title": "Anticancer Res",
"key": "2290_CR31",
"unstructured": "Grant WB. Review of recent advances in understanding the role of vitamin D in reducing cancer risk: breast, colorectal, prostate, and overall cancer. Anticancer Res. 2020;40(1):491–9.",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1080/20009666.2019.1701839",
"author": "T Haykal",
"doi-asserted-by": "crossref",
"first-page": "480",
"issue": "6",
"journal-title": "J Community Hosp Intern Med Perspect",
"key": "2290_CR32",
"unstructured": "Haykal T, Samji V, Zayed Y, Gakhal I, Dhillon H, Kheiri B, et al. The role of vitamin D supplementation for primary prevention of cancer: meta-analysis of randomized controlled trials. J Community Hosp Intern Med Perspect. 2019;9(6):480–8.",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1155/2019/4145821",
"author": "P Perge",
"doi-asserted-by": "crossref",
"first-page": "4145821",
"journal-title": "Dis Markers.",
"key": "2290_CR33",
"unstructured": "Perge P, Boros AM, Gellér L, Osztheimer I, Szilágyi S, Tahin T, et al. Vitamin D deficiency predicts poor clinical outcomes in heart failure patients undergoing cardiac resynchronization therapy. Dis Markers. 2019;2019:4145821.",
"volume": "2019",
"year": "2019"
},
{
"DOI": "10.1016/j.ahj.2010.03.031",
"author": "NC Grandi",
"doi-asserted-by": "crossref",
"first-page": "1044",
"issue": "6",
"journal-title": "Am Heart J",
"key": "2290_CR34",
"unstructured": "Grandi NC, Breitling LP, Vossen CY, Hahmann H, Wüsten B, März W, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. 2010;159(6):1044–51.",
"volume": "159",
"year": "2010"
},
{
"DOI": "10.1056/NEJMoa1809944",
"author": "JE Manson",
"doi-asserted-by": "crossref",
"first-page": "33",
"issue": "1",
"journal-title": "New Engl J Med",
"key": "2290_CR35",
"unstructured": "Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. New Engl J Med. 2019;380(1):33–44.",
"volume": "380",
"year": "2019"
},
{
"DOI": "10.1001/jamacardio.2017.0175",
"author": "R Scragg",
"doi-asserted-by": "crossref",
"first-page": "608",
"issue": "6",
"journal-title": "JAMA Cardiol",
"key": "2290_CR36",
"unstructured": "Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiol. 2017;2(6):608–16.",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.3390/ijms20225811",
"author": "DN Roffe-Vazquez",
"doi-asserted-by": "crossref",
"first-page": "5811",
"issue": "22",
"journal-title": "Int J Mol Sci.",
"key": "2290_CR37",
"unstructured": "Roffe-Vazquez DN, Huerta-Delgado AS, Castillo EC, Villarreal-Calderón JR, Gonzalez-Gil AM, Enriquez C, Garcia-Rivas G, Elizondo-Montemayor L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int J Mol Sci. 2019;20(22):5811.",
"volume": "20",
"year": "2019"
},
{
"author": "MR Pinzone",
"first-page": "1218",
"issue": "9",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "2290_CR38",
"unstructured": "Pinzone MR, Di Rosa M, Malaguarnera M, Madeddu G, Focà E, Ceccarelli G, et al. Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic. Eur Rev Med Pharmacol Sci. 2013;17(9):1218–32.",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.1155/2015/735615",
"author": "P Mansueto",
"doi-asserted-by": "crossref",
"journal-title": "BioMed Res Int.",
"key": "2290_CR39",
"unstructured": "Mansueto P, Seidita A, Vitale G, Gangemi S, Iaria C, Cascio A. Vitamin D deficiency in HIV infection: not only a bone disorder. BioMed Res Int. 2015;2015:735615.",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.2147/IDR.S228336",
"author": "B Ayelign",
"doi-asserted-by": "crossref",
"first-page": "111",
"journal-title": "Infect Drug Resist",
"key": "2290_CR40",
"unstructured": "Ayelign B, Workneh M, Molla MD, Dessie G. Role of vitamin-D supplementation in TB/HIV co-infected patients. Infect Drug Resist. 2020;13:111–8.",
"volume": "13",
"year": "2020"
},
{
"author": "AS Dusso",
"key": "2290_CR41",
"unstructured": "Dusso AS. Molecular biology of Vitamin D: genomic and nongenomic actions of vitamin D in chronic kidney disease. Switzerland: Springer International Publishing Switzerland; 2016.",
"volume-title": "Molecular biology of Vitamin D: genomic and nongenomic actions of vitamin D in chronic kidney disease",
"year": "2016"
},
{
"DOI": "10.1186/s12964-019-0488-2",
"author": "D Liu",
"doi-asserted-by": "crossref",
"first-page": "163",
"issue": "1",
"journal-title": "Cell Commun Signal",
"key": "2290_CR42",
"unstructured": "Liu D, Fang YX, Wu X, Tan W, Zhou W, Zhang Y, et al. 1,25-(OH)(2)D(3)/Vitamin D receptor alleviates systemic lupus erythematosus by downregulating Skp2 and upregulating p27. Cell Commun Signal. 2019;17(1):163.",
"volume": "17",
"year": "2019"
},
{
"DOI": "10.1038/s41467-019-13659-4",
"author": "NC Gassen",
"doi-asserted-by": "crossref",
"first-page": "5770",
"issue": "1",
"journal-title": "Nat Commun",
"key": "2290_CR43",
"unstructured": "Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat Commun. 2019;10(1):5770.",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa1911124",
"author": "AA Ginde",
"doi-asserted-by": "crossref",
"first-page": "2529",
"issue": "26",
"journal-title": "N Engl J Med.",
"key": "2290_CR44",
"unstructured": "Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, et al. Early high-dose vitamin D(3) for critically ill, vitamin D-deficient patients. N Engl J Med. 2019;381(26):2529–40.",
"volume": "381",
"year": "2019"
},
{
"author": "M Metzger",
"key": "2290_CR45",
"unstructured": "Metzger M, Stengel B. Epidemiology of vitamin D deficiency in chronic kidney disease. Switzerland: Springer International Publishing Switzerland; 2016.",
"volume-title": "Epidemiology of vitamin D deficiency in chronic kidney disease",
"year": "2016"
},
{
"DOI": "10.1186/s13063-017-1819-5",
"author": "CR Sudfeld",
"doi-asserted-by": "crossref",
"first-page": "66",
"issue": "1",
"journal-title": "Trials.",
"key": "2290_CR46",
"unstructured": "Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D(3) supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1):66.",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1177/0269216315601954",
"author": "M Martínez-Alonso",
"doi-asserted-by": "crossref",
"first-page": "89",
"issue": "1",
"journal-title": "Palliat Med",
"key": "2290_CR47",
"unstructured": "Martínez-Alonso M, Dusso A, Ariza G, Nabal M. Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: a cross-sectional study. Palliat Med. 2016;30(1):89–96.",
"volume": "30",
"year": "2016"
},
{
"DOI": "10.1038/ki.2008.343",
"author": "M Naves-Diaz",
"doi-asserted-by": "crossref",
"first-page": "1070",
"issue": "8",
"journal-title": "Kidney Int.",
"key": "2290_CR48",
"unstructured": "Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070–8.",
"volume": "74",
"year": "2008"
},
{
"DOI": "10.1001/archinte.168.15.1629",
"author": "ML Melamed",
"doi-asserted-by": "crossref",
"first-page": "1629",
"issue": "15",
"journal-title": "Arch Intern Med.",
"key": "2290_CR49",
"unstructured": "Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37.",
"volume": "168",
"year": "2008"
},
{
"DOI": "10.3945/ajcn.111.014779",
"author": "A Zittermann",
"doi-asserted-by": "crossref",
"first-page": "91",
"issue": "1",
"journal-title": "Am J Clin Nutr",
"key": "2290_CR50",
"unstructured": "Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95(1):91–100.",
"volume": "95",
"year": "2012"
},
{
"author": "R Chowdhury",
"journal-title": "BMJ (Clinical research ed).",
"key": "2290_CR51",
"unstructured": "Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ (Clinical research ed). 2014;348:g1903.",
"volume": "348",
"year": "2014"
},
{
"author": "Y Zhang",
"journal-title": "Bmj.",
"key": "2290_CR52",
"unstructured": "Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. Bmj. 2019;366:l4673.",
"volume": "366",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0170791",
"author": "M Gaksch",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "PLoS One",
"key": "2290_CR53",
"unstructured": "Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12(2):e0170791.",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1001/jama.2020.26848",
"author": "IH Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"issue": "11",
"journal-title": "Jama.",
"key": "2290_CR54",
"unstructured": "Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. Jama. 2021;325(11):1053–60.",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1007/s40618-021-01614-4",
"author": "R Pal",
"doi-asserted-by": "crossref",
"first-page": "53",
"issue": "1",
"journal-title": "J Endocrinol Invest.",
"key": "2290_CR55",
"unstructured": "Pal R, Banerjee M, Bhadada SK, Shetty AJ, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID-19: a systematic review and meta-analysis. J Endocrinol Invest. 2022;45(1):53–68.",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1097/INF.0000000000002225",
"author": "K Hueniken",
"doi-asserted-by": "crossref",
"first-page": "564",
"issue": "6",
"journal-title": "Pediatr Infect Dis J",
"key": "2290_CR56",
"unstructured": "Hueniken K, Aglipay M, Birken CS, Parkin PC, Loeb MB, Thorpe KE, et al. Effect of high-dose vitamin D supplementation on upper respiratory tract infection symptom severity in healthy children. Pediatr Infect Dis J. 2019;38(6):564–8.",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.3945/ajcn.115.113886",
"author": "N Tukvadze",
"doi-asserted-by": "crossref",
"first-page": "1059",
"issue": "5",
"journal-title": "Am J Clin Nutr",
"key": "2290_CR57",
"unstructured": "Tukvadze N, Sanikidze E, Kipiani M, Hebbar G, Easley KA, Shenvi N, et al. High-dose vitamin D3 in adults with pulmonary tuberculosis: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;102(5):1059–69.",
"volume": "102",
"year": "2015"
},
{
"DOI": "10.1007/s10096-018-3306-7",
"author": "L Bjorkhem-Bergman",
"doi-asserted-by": "crossref",
"first-page": "1735",
"issue": "9",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "2290_CR58",
"unstructured": "Bjorkhem-Bergman L, Missailidis C, Karlsson-Valik J, Tammelin A, Ekstrom L, Bottai M, et al. Vitamin D supplementation to persistent carriers of MRSA-a randomized and placebo-controlled clinical trial. Eur J Clin Microbiol Infect Dis. 2018;37(9):1735–44.",
"volume": "37",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa1915176",
"author": "D Ganmaa",
"doi-asserted-by": "crossref",
"first-page": "359",
"issue": "4",
"journal-title": "New Engl J Med",
"key": "2290_CR59",
"unstructured": "Ganmaa D, Uyanga B, Zhou X, Gantsetseg G, Delgerekh B, Enkhmaa D, et al. Vitamin D supplements for prevention of tuberculosis infection and disease. New Engl J Med. 2020;383(4):359–68.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1097/CM9.0000000000000554",
"author": "J Zhang",
"doi-asserted-by": "crossref",
"first-page": "2950",
"issue": "24",
"journal-title": "Chin Med J",
"key": "2290_CR60",
"unstructured": "Zhang J, Chen C, Yang J. Effectiveness of vitamin D supplementation on the outcome of pulmonary tuberculosis treatment in adults: a meta-analysis of randomized controlled trials. Chin Med J. 2019;132(24):2950–9.",
"volume": "132",
"year": "2019"
},
{
"DOI": "10.1093/cid/ciz801",
"author": "CA Camargo",
"doi-asserted-by": "crossref",
"first-page": "311",
"issue": "2",
"journal-title": "Clin Infect Dis",
"key": "2290_CR61",
"unstructured": "Camargo CA, Sluyter J, Stewart AW, Khaw KT, Lawes CMM, Toop L, et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: a randomized controlled trial. Clin Infect Dis. 2020;71(2):311–7.",
"volume": "71",
"year": "2020"
},
{
"author": "AK Kasahara",
"issue": "6",
"journal-title": "PLoS One.",
"key": "2290_CR62",
"unstructured": "Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) serum seasonality in the United States. PLoS One. 2013;8(6):e65785.",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1093/ndt/gfq304",
"author": "M Naves-Diaz",
"doi-asserted-by": "crossref",
"first-page": "1938",
"issue": "6",
"journal-title": "Nephrol Dial Transplant",
"key": "2290_CR63",
"unstructured": "Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernandez-Martin JL, Rodriguez-Puyol D, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26(6):1938–47.",
"volume": "26",
"year": "2011"
},
{
"DOI": "10.1093/ndt/13.suppl_3.41",
"author": "JB Díaz Lopez",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "Suppl 3",
"journal-title": "Nephrol Dial Transplant.",
"key": "2290_CR64",
"unstructured": "Díaz Lopez JB, Jorgetti V, Caorsi H, Ferreira A, Palma A, Menéndez P, et al. Epidemiology of renal osteodystrophy in Iberoamerica. Nephrol Dial Transplant. 1998;13(Suppl 3):41–5.",
"volume": "13",
"year": "1998"
},
{
"DOI": "10.1097/00000441-200008000-00006",
"author": "JL Fernández-Martín",
"doi-asserted-by": "crossref",
"first-page": "96",
"issue": "2",
"journal-title": "Am J Med Sci.",
"key": "2290_CR65",
"unstructured": "Fernández-Martín JL, Canteros A, Alles A, Massari P, Cannata-Andía J. Aluminum exposure in chronic renal failure in iberoamerica at the end of the 1990s: overview and perspectives. Am J Med Sci. 2000;320(2):96–9.",
"volume": "320",
"year": "2000"
}
],
"reference-count": 65,
"references-count": 65,
"relation": {},
"score": 1,
"short-container-title": [
"BMC Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D—a randomised multicentre international clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "20"
}
