High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial
et al., PLoS Medicine, doi:10.1371/journal.pmed.1003999, COVIT-TRIAL, NCT04344041, May 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
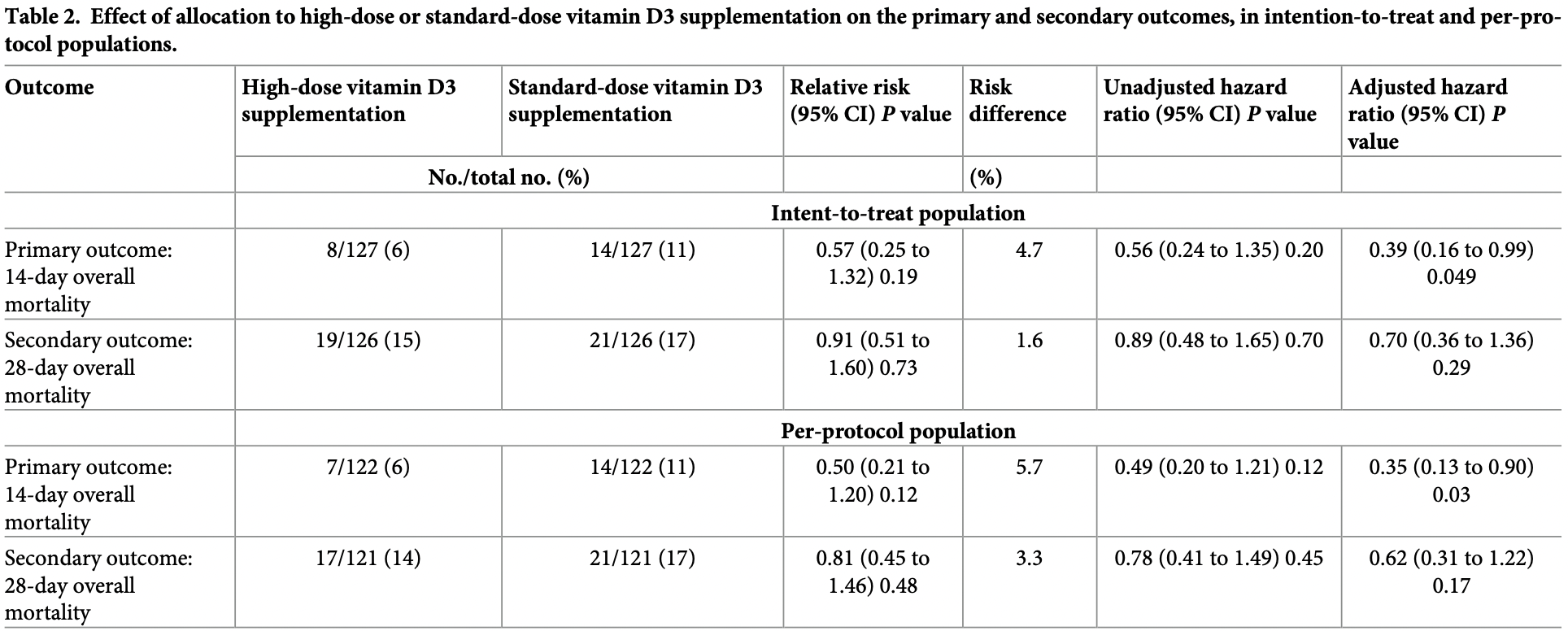
RCT comparing single dose 400,000IU and single dose 50,000IU vitamin D in France, showing lower mortality with the higher dose, statistically significant only at day 14.
The aHR for days 0-5 was 1.30 [0.31-5.35], compared to 0.11 [0.02-0.52] for days 6-14, which in part may reflect the conversion delay for cholecalciferol treatment. The lower efficacy at day 28 vs. day 14 may in part reflect use of only a single dose.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 19th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 85th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
|
risk of death, 30.0% lower, HR 0.70, p = 0.29, treatment 19 of 126 (15.1%), control 21 of 126 (16.7%), Cox proportional hazards, day 28, intention-to-treat.
|
|
risk of death, 38.0% lower, HR 0.62, p = 0.17, treatment 17 of 121 (14.0%), control 21 of 121 (17.4%), Cox proportional hazards, day 28, per-protocol.
|
|
risk of death, 61.0% lower, HR 0.39, p = 0.049, treatment 8 of 127 (6.3%), control 14 of 127 (11.0%), Cox proportional hazards, day 14, intention-to-treat.
|
|
risk of death, 65.0% lower, HR 0.35, p = 0.03, treatment 7 of 122 (5.7%), control 14 of 122 (11.5%), Cox proportional hazards, day 14, per-protocol.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Annweiler et al., 31 May 2022, Randomized Controlled Trial, France, peer-reviewed, 17 authors, study period 15 April, 2020 - 17 December, 2020, dosage 400,000IU single dose, trial NCT04344041 (history) (COVIT-TRIAL).
High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial
PLOS Medicine, doi:10.1371/journal.pmed.1003999
¶ Membership of the COVIT-TRIAL study group is provided in S1 Supplemental Appendix.
Supporting information S1
References
Alcala-Diaz, Limia-Perez, Gomez-Huelgas, Md, Cortes-Rodriguez et al., Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study, Nutrients, doi:10.3390/nu13061760
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial, JAMA, doi:10.1001/jama.2014.13204
Annweiler, Beaudenon, Gautier, Simon, Dube ´e et al., COVID-19 and high-dose VITamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-020-04928-5
Annweiler, Corvaisier, Gautier, Dube ´e, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Annweiler, Hanotte, De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105771
Baktash, Hosack, Patel, Shah, Kandiah et al., Vitamin D status and outcomes for hospitalised older patients with COVID-19, Postgrad Med J, doi:10.1136/postgradmedj-2020-138712
Bandeira, Lazaretti-Castro, Binkley, Clinical aspects of SARS-CoV-2 infection and vitamin D: COVID-19 and the endocrine system: special issue for reviews in endocrine and metabolic disorders, Rev Endocr Metab Disord
Bishop, Ismailova, Dimeloe, Hewison, White, Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory, JBMR Plus, doi:10.1002/jbm4.10405
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Castillo, Costa, Barrios, Alcala ´dı ´az, Lo ´pez Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Covid, -19, Coronavirus Disease
Ct ; Lisewski, computed tomography; eGFR, estimated glomerular filtration rate; IQR, interquartile range; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, doi:10.1136/bmj.n1201
Glinsky, Tripartite Combination of Candidate Pandemic Mitigation Agents: Vitamin D, Quercetin, and Estradiol Manifest Properties of Medicinal Agents for Targeted Mitigation of the COVID-19 Pandemic Defined by Genomics-Guided Tracing of SARS-CoV-2 Targets in Human Cells, Biomedicine
Green, Mcentegart, Byrom, Ghani, Shepherd, Minimization in crossover trials with nonprognostic strata: theory and practical application, J Clin Pharm Ther, doi:10.1046/j.1365-2710.2001.00332.x
Hathcock, Shao, Vieth, Heaney, Risk assessment for vitamin D, Am J Clin Nutr, doi:10.1093/ajcn/85.1.6
Jolliffe, Camargo, Jr, Sluyter, Aglipay et al., Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587%2821%2900051-6
Kong, Zhu, Shi, Liu, Chen et al., VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system, Mol Endocrinol, doi:10.1210/me.2013-1146
Ling, Broad, Murphy, Pappachan, Pardesi-Newton et al., High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study, Nutrients, doi:10.3390/nu12123799
Lingsma, Roozenbeek, Steyerberg, Covariate adjustment increases statistical power in randomized controlled trials, J Clin Epidemiol, doi:10.1016/j.jclinepi.2010.05.003
Manson, Cook, Lee, Bassuk, Mora, Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease, N Engl J Med, doi:10.1056/NEJMoa1809944
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Nogues, Ovejero, Pineda-Moncusı, Bouillon, Arenas et al., Calcifediol Treatment and COVID-19-Related Outcomes, J Clin Endocrinol Metab, doi:10.1210/clinem/dgab405
Pittas, Hughes, Sheehan, Ware, Knowler et al., Vitamin D Supplementation and Prevention of Type 2 Diabetes, N Engl J Med, doi:10.1056/NEJMoa1900906
Rake, Gilham, Bukasa, Ostler, Newton et al., High-dose oral vitamin D supplementation and mortality in people aged 65-84 years: the VIDAL cluster feasibility RCT of open versus double-blind individual randomisation, Health Technol Assess, doi:10.3310/hta24100
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study), Postgrad Med J, doi:10.1136/postgradmedj-2020-139065
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-Week 5000 IU versus 1000 IU Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial, Nutrients, doi:10.3390/nu13072170
Senn, A personal view of some controversies in allocating treatment to patients in clinical trials, Stat Med, doi:10.1002/sim.4780142406
Smet, Smet, Herroelen, Gryspeerdt, Martens, Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality, Am J Clin Pathol, doi:10.1093/ajcp/aqaa252
Tu, Shalay, Pater, Adjustments of treatment effect for covariates in clinical trials: statistical and regulatory issues, Drug Inf J
Vassiliou, Jahaj, Pratikaki, Orfanos, Dimopoulou et al., Low 25-Hydroxyvitamin D Levels on Admission to the Intensive Care Unit May Predispose COVID-19 Pneumonia Patients to a Higher 28-Day Mortality Risk: A Pilot Study on a Greek ICU Cohort, Nutrients
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Xu, Yang, Chen, Luo, Zhang et al., Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system, Mol Med Rep, doi:10.3892/mmr.2017.7546
