Calcifediol Treatment and COVID-19-Related Outcomes
et al., The Journal of Clinical Endocrinology & Metabolism, doi:10.1210/clinem/dgab405, Jan 2021 (preprint)
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Quasi-randomized trial with 930 hospitalized patients, 447 treated with calcifediol, showing significantly lower ICU admission and death with treatment. Note that the randomization in this trial is by ward. Authors report that patients were allocated to empty beds available at admission time regardless of patient conditions, and that staff in all wards followed the same protocol.
The earlier preprint for this article was censored by the Lancet. The Lancet reportedly requested a review from a Twitter user that posted negative comments1. The review provides useful feedback for the authors to improve the reporting of the cluster nature of the RCT, and to explain the delay in registration, however it is highly unusual to censor a preprint in this way. Authors responded to the issues raised here:2
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 19th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 79.0% lower, RR 0.21, p = 0.001, treatment 21 of 447 (4.7%), control 62 of 391 (15.9%), NNT 9.0, adjusted per study, ITT.
|
|
risk of death, 48.0% lower, RR 0.52, p = 0.001, treatment 500, control 338, adjusted per study, including patients treated later.
|
|
risk of ICU admission, 87.0% lower, RR 0.13, p < 0.001, treatment 20 of 447 (4.5%), control 82 of 391 (21.0%), NNT 6.1, adjusted per study, ITT.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Nogués et al., 22 Jan 2021, prospective quasi-randomized (ward), Spain, peer-reviewed, 16 authors, dosage calcifediol 0.5mg day 1, 0.27mg day 3, 0.27mg day 7, 0.27mg day 15, 0.27mg day 30.
Calcifediol treatment and COVID-19-related outcomes
Background COVID-19 is a major health problem because of acute respiratory distress syndrome, saturation of intensive care units (ICU) and mortality.
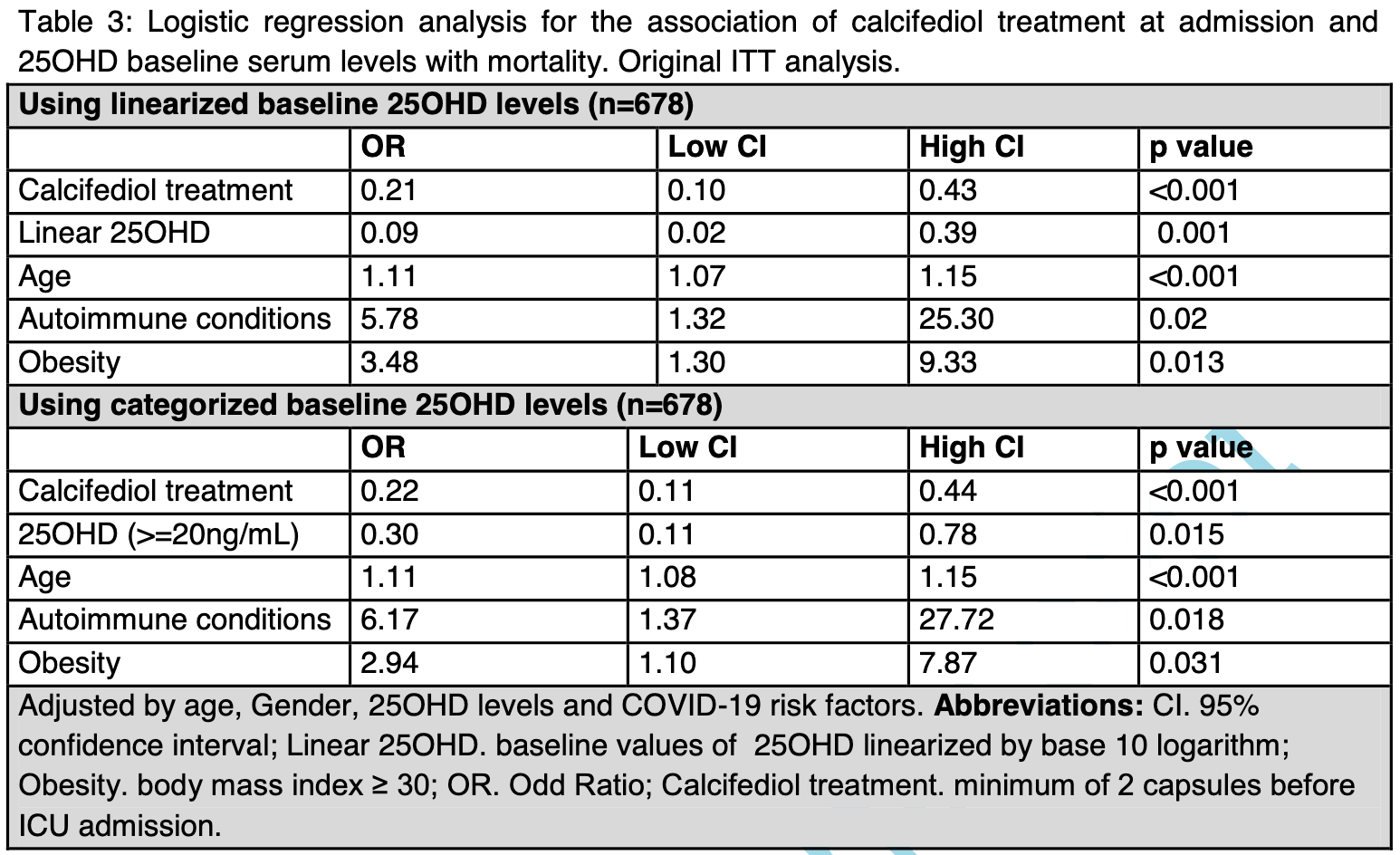
Methods Our study aims to elucidate the effect of calcifediol [25(OH)D 3 ] treatment on ICU admission and mortality, in patients admitted to COVID-19 wards of Hospital del Mar, Barcelona, Spain. A total of 930 participants were included. Participants (n=551) were randomly assigned to calcifediol treatment (532 ug on day one and 266 ug on day 3, 7, 15, and 30) at the time of hospital admission or as controls (n=379). Findings ICU assistance was required by 110 (11.8%) participants. Out of 551 patients treated with calcifediol at admission, 30 (5.4%) required ICU, compared to 80 out of 379 controls (21.1%; p<0.0001). Logistic regression of calcifediol treatment on ICU admission, adjusted by age, gender, linearized 25(OH)D levels at baseline, and comorbidities showed that treated patients had a reduced risk to require ICU (RR 0.18 [95% CI 0.11;0.29]). Baseline 25(OH)D levels inversely correlated with the risk of ICU admission (RR 0.53 [95% CI 0.35;0.80]). Overall mortality was 10%. In the Intention-to-treat analysis, 36 (6.5%) out of 551 patients treated with calcifediol at admission died compared to 57 patients (15%) out of 379 controls (p=0.001). Adjusted results showed a reduced mortality for more of 60%. Higher baseline 25(OH)D levels were significantly associated with decreased mortality (RR 0.40 [95% CI 0.24;0.67]). Age and obesity were also predictors of mortality.
Interpretation In patients hospitalized with COVID-19, calcifediol treatment at the time of hospitalization significantly reduced ICU admission and mortality.
Implications of all the available evidence Vitamin D deficiency is common and even more so in COVID-19 patients compared to the general population. Rapid correction of such deficiency by calcifediol is easy, cheap, and appears as highly effective to control disease severity and avoid fatal outcomes in the setting of SARS-CoV-2 infection.
References
Adorini, Penna, Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists, Hum Immunol
Amrein, Scherkl, Hoffmann, OH)D deficiency 2.0: an update on the current status worldwide, Eur J Clin Nutr
Baktash, Hosack, Patel, Vitamin D status and outcomes for hospitalised older patients with COVID-19, Postgrad Med J
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Boban, Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments, Int J Clin Pract
Bouillon, Marcocci, Carmeliet, Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions, Endocr Rev
Carpagnano, Lecce, Quaranta, Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19, J Endocrinol Invest
Castillo, Costa, Barrios, Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol
Channappanavar, Perlman, Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Semin Immunopathol
Group, Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, N Engl J Med
Guan, Ni, Hu, Clinical Characteristics of Coronavirus Disease 2019 in China, N Engl J Med
Hastie, Pell, Sattar, Vitamin D and COVID-19 infection and mortality in UK Biobank, Eur J Nutr
Helming, Bose, Ehrchen, 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation, Blood
Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin Exp Res
Jordan, Adab, Cheng, Covid-19: risk factors for severe disease and death, BMJ
Kalil, Patterson, Mehta, Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N Engl J Med
Maghbooli, Sahraian, Ebrahimi, Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS One
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw Open
Nair, Venkatesh, Center, Vitamin D deficiency and supplementation in critical illness-the known knowns and known unknowns, Crit Care
Nirula, Shen, Skovronsky, Neutralizing Antibody LY-CoV555 for Outpatient Covid-19. Reply, N Engl J Med
Panagiotou, Tee, Ihsan, Original publication: Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity, Clin Endocrinol (Oxf)
Pizzini, Aichner, Sahanic, Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry, Nutrients
Quesada-Gomez, Bouillon, Is calcifediol better than cholecalciferol for vitamin D supplementation?, Osteoporos Int
Radujkovic, Hippchen, Tiwari-Heckler, Dreher, Boxberger, Vitamin D Deficiency and Outcome of COVID-19 Patients, Nutrients
Russo, Pitter, Da Re, Tonon, Avossa et al., Epidemiology and public health response in early phase of COVID-19 pandemic, Veneto Region, Italy, 21 February to, Euro Surveill
Xu, Shi, Wang, Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med
Ye, Tang, Liao, Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?-A Case-Control Study, J Am Coll Nutr
DOI record:
{
"DOI": "10.1210/clinem/dgab405",
"ISSN": [
"0021-972X",
"1945-7197"
],
"URL": "http://dx.doi.org/10.1210/clinem/dgab405",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Context</jats:title>\n <jats:p>COVID-19 is a major health problem because of saturation of intensive care units (ICU) and mortality. Vitamin D has emerged as a potential treatment able to reduce the disease severity.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>This work aims to elucidate the effect of 25(OH)D3 (calcifediol) treatment on COVID-19–related outcomes.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This observational cohort study was conducted from March to May 2020, among patients admitted to COVID-19 wards of Hospital del Mar, Barcelona, Spain. A total of 930 patients with COVID-19 were included; 92 were excluded because of previous calcifediol intake. Of the remaining 838, a total of 447 received calcifediol (532 μg on day 1 plus 266 μg on days 3, 7, 15, and 30), whereas 391 were not treated at the time of hospital admission (intention-to-treat). Of the latter, 53 patients were treated later during ICU admission and were allocated in the treated group in a second analysis. In healthy individuals, calcifediol is about 3.2-fold more potent on a weight basis than cholecalciferol. Main outcome measures were ICU admission and mortality.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>ICU assistance was required by 102 (12.2%) participants. Out of 447 patients treated with calcifediol at admission, 20 (4.5%) required the ICU, compared to 82 (21%) out of 391 nontreated (P &lt; .001). Logistic regression of calcifediol treatment on ICU admission, adjusted by age, sex, linearized 25-hydroxyvitamin D levels at baseline, and comorbidities showed that treated patients had a reduced risk of requiring the ICU (odds ratio [OR] 0.13; 95% CI 0.07-0.23). Overall mortality was 10%. In the intention-to-treat analysis, 21 (4.7%) out of 447 patients treated with calcifediol at admission died compared to 62 patients (15.9%) out of 391 nontreated (P = .001). Adjusted results showed a reduced mortality risk with an OR of 0.21 (95% CI, 0.10-0.43). In the second analysis, the obtained OR was 0.52 (95% CI, 0.27-0.99).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>In patients hospitalized with COVID-19, calcifediol treatment significantly reduced ICU admission and mortality.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
},
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Nogues",
"given": "Xavier",
"sequence": "first"
},
{
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
},
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Ovejero",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
}
],
"family": "Pineda-Moncusí",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6446-3763",
"affiliation": [
{
"name": "Clinical and Experimental Endocrinology, Department of Chronic Diseases and Metabolism, KU Leuven, Herestraat, 3000 Leuven, Belgium"
}
],
"authenticated-orcid": false,
"family": "Bouillon",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital del Mar–IMIM, Barcelona 08003, Spain"
}
],
"family": "Arenas",
"given": "Dolors",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Nephrology, Hospital del Mar–IMIM, Barcelona 08003, Spain"
}
],
"family": "Pascual",
"given": "Julio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
}
],
"family": "Ribes",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
},
{
"name": "Department of Infectious Diseases, Hospital del Mar–IMIM, Barcelona 08003,Spain"
}
],
"family": "Guerri-Fernandez",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Hospital del Mar–IMIM, Barcelona 08003,Spain"
}
],
"family": "Villar-Garcia",
"given": "Judit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Rial",
"given": "Abora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Gimenez-Argente",
"given": "Carme",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Cos",
"given": "Maria Lourdes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Rodriguez-Morera",
"given": "Jaime",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Hospital del Mar, Universitat Autònoma de Barcelona, Barcelona 08003, Spain"
}
],
"family": "Campodarve",
"given": "Isabel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Maimónides de Investigación Biomédica de Córdoba (IMIBIC), Fundación Progreso y Salud, CIBER de Fragilidad y Envejecimiento Saludable (CIBERFES), Hospital Universitario Reina Sofía, Universidad de Córdoba, 14004, Córdoba, Spain"
}
],
"family": "Quesada-Gomez",
"given": "José Manuel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6507-0147",
"affiliation": [
{
"name": "IMIM (Hospital del Mar Medical Research Institute), Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Barcelona 08003, Spain"
}
],
"authenticated-orcid": false,
"family": "Garcia-Giralt",
"given": "Natalia",
"sequence": "additional"
}
],
"container-title": "The Journal of Clinical Endocrinology & Metabolism",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
7
]
],
"date-time": "2021-06-07T14:51:41Z",
"timestamp": 1623077501000
},
"deposited": {
"date-parts": [
[
2021,
11,
6
]
],
"date-time": "2021-11-06T12:00:52Z",
"timestamp": 1636200052000
},
"funder": [
{
"DOI": "10.13039/100012619",
"award": [
"CB16/10/00245",
"CB16/10/00501"
],
"doi-asserted-by": "publisher",
"name": "Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable"
},
{
"award": [
"PI19/00033"
],
"name": "Formación en Investigación en Salud"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:02:03Z",
"timestamp": 1712070123616
},
"is-referenced-by-count": 55,
"issue": "10",
"issued": {
"date-parts": [
[
2021,
6,
7
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2021,
6,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
9,
27
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/journals/pages/open_access/funder_policies/chorus/standard_publication_model",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
7
]
],
"date-time": "2021-06-07T00:00:00Z",
"timestamp": 1623024000000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/jcem/advance-article-pdf/doi/10.1210/clinem/dgab405/39552930/dgab405.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jcem/article-pdf/106/10/e4017/41098012/dgab405.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jcem/article-pdf/106/10/e4017/41098012/dgab405.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "80",
"original-title": [],
"page": "e4017-e4027",
"prefix": "10.1210",
"published": {
"date-parts": [
[
2021,
6,
7
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
7
]
]
},
"published-other": {
"date-parts": [
[
2021,
10,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
9,
27
]
]
},
"publisher": "The Endocrine Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"issue": "18",
"journal-title": "N Engl J Med.",
"key": "2021110611591708600_CIT0001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1111/ijcp.13868",
"article-title": "Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments",
"author": "Boban",
"doi-asserted-by": "crossref",
"first-page": "e13868",
"issue": "4",
"journal-title": "Int J Clin Pract.",
"key": "2021110611591708600_CIT0002",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.47.2000548",
"article-title": "Epidemiology and public health response in early phase of COVID-19 pandemic, Veneto Region, Italy, 21 February to 2 April 2020",
"author": "Russo",
"doi-asserted-by": "crossref",
"first-page": "2000548",
"issue": "47",
"journal-title": "Euro Surveill",
"key": "2021110611591708600_CIT0003",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med.",
"key": "2021110611591708600_CIT0004",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19—preliminary report",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med.",
"key": "2021110611591708600_CIT0005",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med.",
"key": "2021110611591708600_CIT0006",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2033787",
"article-title": "Neutralizing antibody LY-CoV555 for outpatient Covid-19. Reply",
"author": "Nirula",
"doi-asserted-by": "crossref",
"first-page": "189",
"issue": "2",
"journal-title": "N Engl J Med.",
"key": "2021110611591708600_CIT0007",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"article-title": "The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality",
"author": "Ilie",
"doi-asserted-by": "crossref",
"first-page": "1195",
"issue": "7",
"journal-title": "Aging Clin Exp Res.",
"key": "2021110611591708600_CIT0008",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"article-title": "Association of vitamin D status and other clinical characteristics with COVID-19 test results",
"author": "Meltzer",
"doi-asserted-by": "crossref",
"first-page": "e2019722",
"issue": "9",
"journal-title": "JAMA Netw Open.",
"key": "2021110611591708600_CIT0009",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1002/jbm4.10405",
"article-title": "Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory",
"author": "Bishop",
"doi-asserted-by": "crossref",
"first-page": "e10405",
"issue": "1",
"journal-title": "JBMR Plus",
"key": "2021110611591708600_CIT0010",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1111/joim.12777",
"article-title": "Vitamin D and tuberculosis: where next?",
"author": "Brighenti",
"doi-asserted-by": "crossref",
"journal-title": "J Intern Med",
"key": "2021110611591708600_CIT0011",
"year": "2018"
},
{
"DOI": "10.1016/j.jsbmb.2018.11.013",
"article-title": "Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells",
"author": "Greiller",
"doi-asserted-by": "crossref",
"first-page": "152",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2021110611591708600_CIT0012",
"volume": "187",
"year": "2019"
},
{
"DOI": "10.1136/bmj.i6583",
"article-title": "Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data",
"author": "Martineau",
"doi-asserted-by": "crossref",
"first-page": "i6583",
"journal-title": "BMJ.",
"key": "2021110611591708600_CIT0013",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1186/s13054-018-2185-8",
"article-title": "Vitamin D deficiency and supplementation in critical illness-the known knowns and known unknowns",
"author": "Nair",
"doi-asserted-by": "crossref",
"first-page": "276",
"issue": "1",
"journal-title": "Crit Care.",
"key": "2021110611591708600_CIT0014",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.3390/nu12040988",
"article-title": "Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths",
"author": "Grant",
"doi-asserted-by": "crossref",
"first-page": "988",
"issue": "4",
"journal-title": "Nutrients",
"key": "2021110611591708600_CIT0015",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"article-title": "Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials",
"author": "Jolliffe",
"doi-asserted-by": "crossref",
"first-page": "276",
"issue": "5",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "2021110611591708600_CIT0016",
"volume": "9",
"year": "2021"
},
{
"author": "World Health Organization",
"key": "2021110611591708600_CIT0017"
},
{
"DOI": "10.1210/er.2018-00126",
"article-title": "Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions",
"author": "Bouillon",
"doi-asserted-by": "crossref",
"first-page": "1109",
"issue": "4",
"journal-title": "Endocr Rev.",
"key": "2021110611591708600_CIT0018",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.1038/s41430-020-0558-y",
"article-title": "Vitamin D deficiency 2.0: an update on the current status worldwide",
"author": "Amrein",
"doi-asserted-by": "crossref",
"first-page": "1498",
"issue": "11",
"journal-title": "Eur J Clin Nutr.",
"key": "2021110611591708600_CIT0019",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "crossref",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2021110611591708600_CIT0020",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial",
"author": "Murai",
"doi-asserted-by": "crossref",
"first-page": "1053",
"issue": "11",
"journal-title": "JAMA.",
"key": "2021110611591708600_CIT0021",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"article-title": "Vitamin D and survival in COVID-19 patients: a quasi-experimental study",
"author": "Annweiler",
"doi-asserted-by": "crossref",
"first-page": "105771",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2021110611591708600_CIT0022",
"volume": "204",
"year": "2020"
},
{
"DOI": "10.18632/aging.202307",
"article-title": "Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests",
"author": "Cangiano",
"doi-asserted-by": "crossref",
"first-page": "24522",
"issue": "24",
"journal-title": "Aging (Albany NY).",
"key": "2021110611591708600_CIT0023",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/nu12123799",
"article-title": "High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study",
"author": "Ling",
"doi-asserted-by": "crossref",
"first-page": "3799",
"issue": "12",
"journal-title": "Nutrients",
"key": "2021110611591708600_CIT0024",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1007/s00198-018-4520-y",
"article-title": "Is calcifediol better than cholecalciferol for vitamin D supplementation?",
"author": "Quesada-Gomez",
"doi-asserted-by": "crossref",
"first-page": "1697",
"issue": "8",
"journal-title": "Osteoporos Int.",
"key": "2021110611591708600_CIT0025",
"volume": "29",
"year": "2018"
},
{
"DOI": "10.1016/j.bone.2013.10.014",
"article-title": "Pharmacokinetics of oral vitamin D3 and calcifediol",
"author": "Jetter",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Bone.",
"key": "2021110611591708600_CIT0026",
"volume": "59",
"year": "2014"
},
{
"DOI": "10.1007/s40618-020-01370-x",
"article-title": "Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19",
"author": "Carpagnano",
"doi-asserted-by": "crossref",
"first-page": "765",
"issue": "4",
"journal-title": "J Endocrinol Invest.",
"key": "2021110611591708600_CIT0027",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0239799",
"article-title": "Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection",
"author": "Maghbooli",
"doi-asserted-by": "crossref",
"first-page": "e0239799",
"issue": "9",
"journal-title": "PloS One.",
"key": "2021110611591708600_CIT0028",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/nu12092757",
"article-title": "Vitamin D deficiency and outcome of COVID-19 patients",
"author": "Radujkovic",
"doi-asserted-by": "crossref",
"first-page": "2757",
"issue": "9",
"journal-title": "Nutrients",
"key": "2021110611591708600_CIT0029",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/07315724.2020.1826005",
"article-title": "Does serum vitamin D level affect COVID-19 infection and its severity?—a case-control study",
"author": "Ye",
"doi-asserted-by": "crossref",
"journal-title": "J Am Coll Nutr",
"key": "2021110611591708600_CIT0030",
"year": "2020"
},
{
"DOI": "10.1111/cen.14310",
"article-title": "Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity",
"author": "Panagiotou",
"doi-asserted-by": "crossref",
"first-page": "629",
"issue": "5",
"journal-title": "Clin Endocrinol (Oxf).",
"key": "2021110611591708600_CIT0031",
"volume": "93",
"year": "2020"
},
{
"DOI": "10.1136/postgradmedj-2020-138712",
"article-title": "Vitamin D status and outcomes for hospitalised older patients with COVID-19",
"author": "Baktash",
"doi-asserted-by": "crossref",
"journal-title": "Postgrad Med J",
"key": "2021110611591708600_CIT0032",
"year": "2020"
},
{
"DOI": "10.1007/s00394-020-02372-4",
"article-title": "Vitamin D and COVID-19 infection and mortality in UK Biobank",
"author": "Hastie",
"doi-asserted-by": "crossref",
"first-page": "545",
"issue": "1",
"journal-title": "Eur J Nutr.",
"key": "2021110611591708600_CIT0033",
"volume": "60",
"year": "2021"
},
{
"DOI": "10.3390/nu12092775",
"article-title": "Impact of vitamin D deficiency on COVID-19—a prospective analysis from the CovILD registry",
"author": "Pizzini",
"doi-asserted-by": "crossref",
"first-page": "2775",
"issue": "9",
"journal-title": "Nutrients",
"key": "2021110611591708600_CIT0034",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"issue": "4",
"journal-title": "Lancet Respir Med.",
"key": "2021110611591708600_CIT0035",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"article-title": "Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology",
"author": "Channappanavar",
"doi-asserted-by": "crossref",
"first-page": "529",
"issue": "5",
"journal-title": "Semin Immunopathol.",
"key": "2021110611591708600_CIT0036",
"volume": "39",
"year": "2017"
},
{
"DOI": "10.1016/j.humimm.2009.01.016",
"article-title": "Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists",
"author": "Adorini",
"doi-asserted-by": "crossref",
"first-page": "345",
"issue": "5",
"journal-title": "Hum Immunol.",
"key": "2021110611591708600_CIT0037",
"volume": "70",
"year": "2009"
},
{
"DOI": "10.1182/blood-2005-03-1029",
"article-title": "1α,25-Dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation",
"author": "Helming",
"doi-asserted-by": "crossref",
"first-page": "4351",
"issue": "13",
"journal-title": "Blood.",
"key": "2021110611591708600_CIT0038",
"volume": "106",
"year": "2005"
},
{
"DOI": "10.1016/j.jsbmb.2020.105719",
"article-title": "Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: revised Ms SBMB 2020_166",
"author": "Quesada-Gomez",
"doi-asserted-by": "crossref",
"first-page": "105719",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "2021110611591708600_CIT0039",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1198",
"article-title": "Covid-19: risk factors for severe disease and death",
"author": "Jordan",
"doi-asserted-by": "crossref",
"first-page": "m1198",
"journal-title": "BMJ.",
"key": "2021110611591708600_CIT0040",
"volume": "368",
"year": "2020"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jcem/article/106/10/e4017/6294179"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Biochemistry (medical)",
"Clinical Biochemistry",
"Endocrinology",
"Biochemistry",
"Endocrinology, Diabetes and Metabolism"
],
"subtitle": [],
"title": "Calcifediol Treatment and COVID-19–Related Outcomes",
"type": "journal-article",
"volume": "106"
}
