A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications
et al., Scientific Reports, doi:10.1038/s41598-024-64260-9, Jun 2024
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
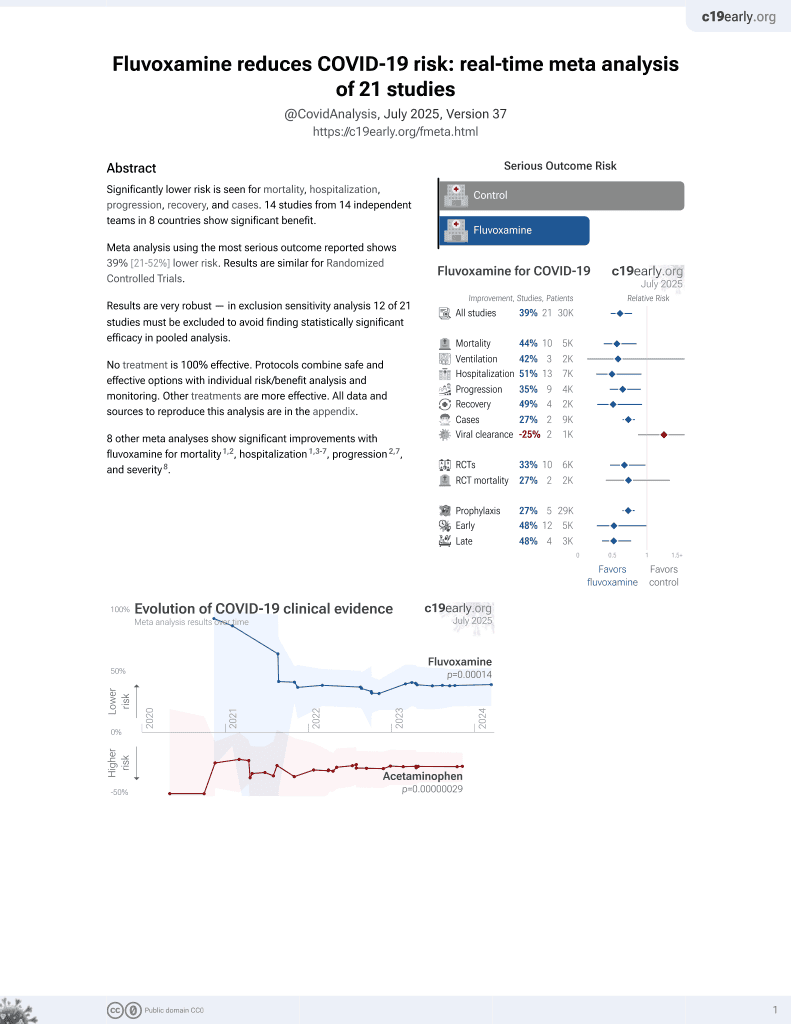
Systematic review and meta analysis of 14 studies showing significantly lower COVID-19 clinical deterioration and mortality with fluvoxamine treatment. Subgroup analysis indicated that higher doses (≥200mg/day) and earlier treatment (within 3 days) provided the greatest benefit in preventing clinical deterioration.
The mortality result is exaggerated due to the use of per-protocol results for Together which has clear confounding (per-protocol defined as 80% adherence)1, and the Siripongboonsitti et al. mortality result is missing, however these do not change the positive result.
9 meta-analyses show significant improvements with fluvoxamine for mortality3-5,
hospitalization3,6-10 ,
progression4,10, and
severity11.
Currently there are 21 fluvoxamine for COVID-19 studies, showing 44% lower mortality [15‑63%], 42% lower ventilation [-151‑86%], 10% higher ICU admission [-72‑326%], 51% lower hospitalization [8‑73%], and 27% fewer cases [18‑35%].
|
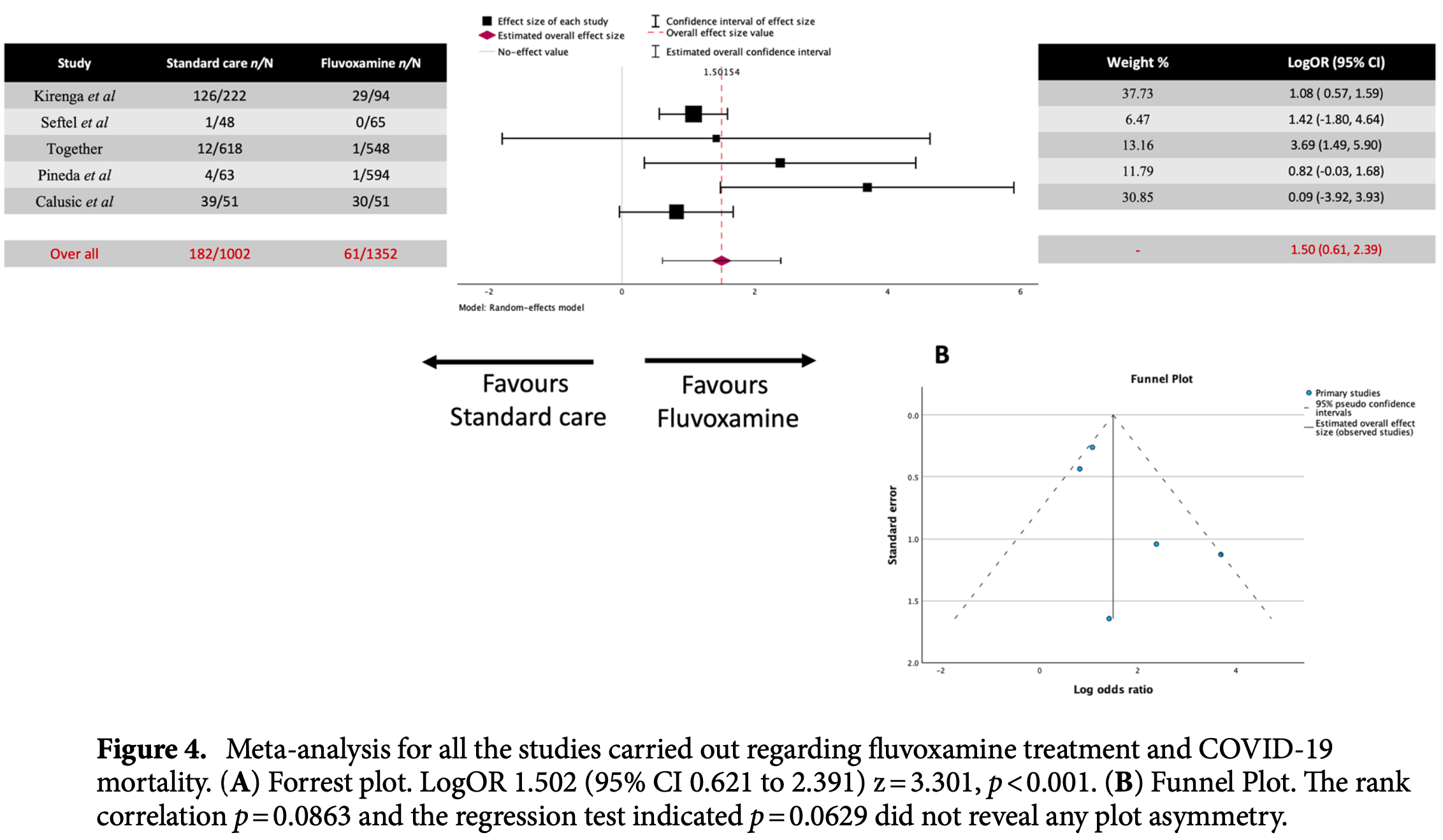
risk of death, 77.7% lower, OR 0.22, p < 0.001, inverted to make OR<1 favor treatment, RR approximated with OR.
|
|
clinical deterioration, 63.6% lower, OR 0.36, p = 0.02, inverted to make OR<1 favor treatment, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Reis et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, The Lancet Global Health, doi:10.1016/S2214-109X(21)00448-4.
2.
Siripongboonsitti et al., The Real-World Effectiveness of Fluvoxamine Therapy in Mild to Moderate COVID-19 Patients; a Historical Cohort Study (Fluvoxa Trial), Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.10.010.
3.
Deng et al., Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: A systematic review and meta-analysis, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.01.010.
4.
Prasanth et al., A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications, Scientific Reports, doi:10.1038/s41598-024-64260-9.
5.
Fico et al., Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis, European Neuropsychopharmacology, doi:10.1016/j.euroneuro.2022.10.004.
6.
Lee et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.6269.
7.
Lu et al., Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis, Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.10.010.
8.
Marcec et al., A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference, Journal of Infection, doi:10.1016/j.jinf.2022.11.011.
9.
Deng (B) et al., Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis, Reviews in Medical Virology, doi:10.1002/rmv.2501.
Prasanth et al., 12 Jun 2024, peer-reviewed, 8 authors.
A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications
Scientific Reports, doi:10.1038/s41598-024-64260-9
There have been 774,075,242 cases of COVID-19 and 7,012,986 deaths worldwide as of January 2024. In the early stages of the pandemic, there was an urgent need to reduce the severity of the disease and prevent the need for hospitalization to avoid stress on healthcare systems worldwide. The repurposing of drugs to prevent clinical deterioration of COVID-19 patients was trialed in many studies using many different drugs. Fluvoxamine (an SSRI and sigma-1 receptor agonist) was initially identified to potentially provide beneficial effects in COVID-19-infected patients, preventing clinical deterioration and the need for hospitalization. Fourteen clinical studies have been carried out to date, with seven of those being randomized placebo-controlled studies. This systematic review and meta-analysis covers the literature from the outbreak of SARS-CoV-2 in late 2019 until January 2024. Search terms related to fluvoxamine, such as its trade names and chemical names, along with words related to COVID-19, such as SARS-CoV-2 and coronavirus, were used in literature databases including PubMed, Google Scholar, Scopus, and the ClinicalTrials.gov database from NIH, to identify the trials used in the subsequent analysis. Clinical deterioration and death data were extracted from these studies where available and used in the meta-analysis. A total of 7153 patients were studied across 14 studies (both open-label and double-blind placebo-controlled). 681 out of 3553 (19.17%) in the standard care group and 255 out of 3600 (7.08%) in the fluvoxamine-treated group experienced clinical deterioration. The estimated average log odds ratio was 1.087 (95% CI 0.200 to 1.973), which differed significantly from zero (z = 2.402, p = 0.016). The seven placebo-controlled studies resulted in a log odds ratio of 0.359 (95% CI 0.1111 to 0.5294), which differed significantly from zero (z = 3.103, p = 0.002). The results of this study identified fluvoxamine as effective in preventing clinical deterioration, and subgrouping OPEN
Author contributions
Competing interests AMR is listed as an inventor on a patent application related to methods of treating COVID-19 (including Sigma1 agonists and specifically fluvoxamine), which was filed by Washington University in St. Louis. No other author declares any potential conflict of interest or competing financial or non-financial interest in relation to the manuscript. AMR is listed on a patent application that includes the use of σ1R agonists for the treatment of COVID-19. No other authors have any conflicts to declare.
References
Batool, Efficacy and safety of favipiravir in treating COVID-19 patients: A meta-analysis of randomized control trials, Cureus
Bramante, Outpatient treatment of Covid-19 with metformin, ivermectin, and fluvoxamine and the development of Long Covid over 10-month follow-up, medRxiv, doi:10.1101/2022.12.21.22283753
Bramante, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2201662
Brimson, The effectiveness of Bacopa monnieri (Linn.) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: analysis of the available clinical data, Sci. Rep
Brown, Pathophysiology, diagnosis, and management of neuroinflammation in covid-19
Calusic, Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls, Br. J. Clin. Pharmacol, doi:10.1111/bcp.15126
Chen, Neuroimmunological effect of vitamin D on neuropsychiatric Long COVID syndrome: A review, Nutrients
Davis, Mccorkell, Vogel, Topol, Long, Major findings, mechanisms and recommendations, Nat. Rev. Microbiol
Demisch, Melatonin and cortisol increase after fluvoxamine, Br. J. Clin. Pharmacol, doi:10.1111/j.1365-2125.1986.tb02947.x
Farahani, Ajam, Naeini, Effect of fluvoxamine on preventing neuropsychiatric symptoms of post COVID syndrome in mild to moderate patients, a randomized placebo-controlled double-blind clinical trial, BMC Infect. Dis, doi:10.1186/s12879-023-08172-5
Feehan, Apostolopoulos, Is COVID-19 the worst pandemic?, Maturitas, doi:10.1016/j.maturitas.2021.02.001
Fenton, Lee, Antidepressants with anti-inflammatory properties may be useful in long COVID depression, Drugs Therapy Perspect
French, Impact of hospital strain on excess deaths during the COVID-19 pandemic-United States, July 2020-July 2021, Morb. Mortal. Wkly. Rep, doi:10.1038/s41598-024-64260-9www.nature.com/scientificreports/
Friesland, Mingorance, Chung, Chisari, Gastaminza, Sigma-1 receptor regulates early steps of viral RNA replication at the onset of hepatitis C virus infection, J. Virol
Fung, Liu, Coronavirus infection, ER stress, apoptosis and innate immunity, Front. Microbiol
Fung, Liu, The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus, Virology, doi:10.1016/j.virol.2019.05.002
Gatti, Ilamathi, Todkar, Germain, Mitochondria targeted viral replication and survival strategies-Prospective on SARS-CoV-2, Front. Pharmacol
Gordon, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Gordon, Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms, Science, doi:10.1126/science.abe9403
Griffin, The importance of understanding the stages of COVID-19 in treatment and trials, AIDS Rev
Groff, Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review, JAMA Netw. Open
Hashimoto, Overview of the potential use of fluvoxamine for COVID-19 and long COVID, Discov. Ment. Health, doi:10.1007/s44192-023-00036-3
Hashimoto, Overview of the potential use of fluvoxamine for COVID-19 and long COVID, Discov. Mental Health, doi:10.1038/s41598-024-64260-9www.nature.com/scientificreports/
Hashimoto, Suzuki, Hashimoto, Comments to "Fluvoxamine and long COVID-19: A new role for sigma-1 receptor (S1R) agonists" by Khani and Entezari-Maleki, Mol. Psychiatry
Hashimoto, Suzuki, Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: A historical review, Mol. Psychiatry
Härtter, Grözinger, Weigmann, Röschke, Hiemke, Increased bioavailability of oral melatonin after fluvoxamine coadministration, Clin. Pharmacol. Ther
Ishikawa, High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: A positron emission tomography study using [11C] SA4503, Biol. Psychiatry
Ishima, Fujita, Hashimoto, Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells, Eur. J. Pharmacol
Jadad, Assessing the quality of reports of randomized clinical trials: Is blinding necessary?, Controlled Clin. Trials
Khani, Entezari-Maleki, Fluvoxamine and long COVID-19: A new role for sigma-1 receptor (S1R) agonists, Mol. Psychiatry
Kirenga, Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: A prospective interventional open-label cohort study, Mol. Psychiatry, doi:10.1038/s41380-023-02004-3
Knoops, SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum, PLoS Biol
Kugler, Klein, Zanger, MiR-155 and other microRNAs downregulate drug metabolizing cytochromes P450 in inflammation, Biochem. Pharmacol, doi:10.1016/j.bcp.2019.113725
Lenze, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2020.22760
Maier, Infectious bronchitis virus generates spherules from zippered endoplasmic reticulum membranes, MBio
Mccarthy, Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: A randomized clinical trial, JAMA, doi:10.1001/jama.2022.24100
Merad, Martin, Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages, Nat. Rev. Immunol
Omori, Fluvoxamine versus other anti-depressive agents for depression, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD006114.pub2
Oskotsky, Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.33090
Ozonoff, Features of acute COVID-19 associated with post-acute sequelae of SARS-CoV-2 phenotypes: Results from the IMPACC study, Nat. Commun, doi:10.1038/s41467-023-44090-5
Papadopoulos, Papadopoulou, Aw, Anexelekto, AXL) no more: microRNA-155 (miR-155) controls the "Uncontrolled" in SARS-CoV-2, Hum. Cell, doi:10.1007/s13577-024-01041-6
Papadopoulos, Papadopoulou, Aw, Beauty and the beast: Host microRNA-155 versus SARS-CoV-2, Hum Cell, doi:10.1007/s13577-023-00867-w
Papadopoulos, Papadopoulou, Aw, Selective serotonin reuptake inhibitors may influence COVID-19 prognosis through antioxidant and cytoprotective pathways mediated by sigma 1 receptor agonism, Pharmacopsychiatry, doi:10.1055/a-1909-2198
Papadopoulos, Sutheesophon, Aw, Anti-SARS-CoV-2 action of fluvoxamine may be mediated by endothelial nitric oxide synthase, Pharmacopsychiatry, doi:10.1055/a-1641-0357
Pedersen, Ho, SARS-CoV-2: a Storm is raging, J. Clin. Investig
Pineda, Impact of fluvoxamine on outpatient treatment of COVID-19 in Honduras in a prospective observational realworld study, Front. Pharmacol, doi:10.3389/fphar.2022.1054644
Puelles, Multiorgan and renal tropism of SARS-CoV-2, N. Engl. J. Med
Rad, Wannigama, Hirankarn, Mclellan, The impact of non-synonymous mutations on miRNA binding sites within the SARS-CoV-2 NSP3 and NSP4 genes, Sci. Rep
Reggiori, Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication, Cell Host Microbe
Reiersen, The STOP COVID 2 Study: Fluvoxamine vs placebo for outpatients with symptomatic COVID-19, a fully remote randomized controlled trial, Open Forum Infect. Dis, doi:10.1093/ofid/ofad419
Reiersen, Zorumski, Lenze, Fluvoxamine and long COVID: Post-acute recovery, Rev. Med. Virol, doi:10.1002/rmv.2557
Reis, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial, Lancet Glob. Health, doi:10.1016/s2214-109x(21)00448-4
Reis, Oral fluvoxamine with inhaled budesonide for treatment of early-onset COVID-19: A randomized platform trial, Ann. Intern. Med
Rhea, The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice, Nat. Neurosci
Rodebaugh, Acute symptoms of mild to moderate COVID-19 are highly heterogeneous across individuals and over time, Open Forum Infect Dis, doi:10.1093/ofid/ofab090
Rosen, Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis, Sci. Transl. Med, doi:10.1126/scitranslmed.aau5266
Seftel, Boulware, Prospective Cohort of fluvoxamine for early treatment of coronavirus disease 19, Open Forum Infect. Dis, doi:10.1093/ofid/ofab050
Seo, Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: A Preliminary result of randomized controlled trial, Infect. Chemother, doi:10.3947/ic.2021.0142
Shah, Favipiravir in patients hospitalised with COVID-19 (PIONEER trial): A multicentre, open-label, phase 3, randomised controlled trial of early intervention versus standard care, Lancet Respir. Med
Sidky, Assessing the effect of selective serotonin reuptake inhibitors in the prevention of post-acute sequelae of COVID-19, medRxiv
Singh, Chaubey, Chen, Suravajhala, Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis, Am. J. Physiol. Cell Physiol
Siripongboonsitti, Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study), Int. J. Infect. Dis, doi:10.1016/j.ijid.2023.06.018
Song, Li, Xie, Hou, You, Cytokine storm induced by SARS-CoV-2, Clin. Chim. Acta
Stewart, Higher-Dose fluvoxamine and time to sustained recovery in outpatients with COVID-19: The ACTIV-6 randomized clinical trial, JAMA, doi:10.1001/jama.2023.23363
Suryasa, Rodríguez-Gámez, Koldoris, The COVID-19 pandemic, Int. J. Health Sci
Tay, Poh, Rénia, Macary, Ng, The trinity of COVID-19: immunity, inflammation and intervention, Nat. Rev. Immunol
Trkulja, Why we should not recommend or offer fluvoxamine to COVID-19 patients?, Eur. J. Clin. Pharmacol, doi:10.1007/s00228-022-03447-3
Tsiakalos, Ziakas, Polyzou, Schinas, Akinosoglou, Early fluvoxamine reduces the risk for clinical deterioration in symptomatic outpatients with COVID-19: A real-world, retrospective, before-after analysis, Microorganisms, doi:10.3390/microorganisms11082073
Vasallo, Gastaminza, Cellular stress responses in hepatitis C virus infection: Mastering a two-edged sword, Virus Res
Vela, Repurposing sigma-1 receptor ligands for COVID-19 therapy?, Front. Pharmacol, doi:10.3389/fphar.2020.582310
Viechtbauer, Bias and efficiency of meta-analytic variance estimators in the random-effects model, J. Educ. Behav. Statistics
Wang, MiR-155 is involved in major depression disorder and antidepressant treatment via targeting SIRT1, Biosci Rep, doi:10.1042/bsr20181139
Wannigama, COVID-19 monitoring with sparse sampling of sewered and non-sewered wastewater in urban and rural communities, Iscience
Wannigama, Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: A randomized clinical trial, EClinicalMedicine
Wannigama, Jacquet, NOD2-dependent BCG-induced trained immunity: A way to regulate innate responses to SARS-CoV2?, Int. J. Infect. Dis
Wannigama, Tracing the new SARS-CoV-2 variant BA. 2.86 in the community through wastewater surveillance in Bangkok, Thailand, Lancet Infect. Dis