Efficacy of Combination Therapy of Fluvoxamine and Favipiravir versus Favipiravir Monotherapy to Prevent Severe COVID-19 among Mild to Moderate COVID-19 Patients: Open-label Randomized Controlled Trial (EFFaCo Study)
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2023.06.018, EFFaCo, TCTR20210615002, Jun 2023
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
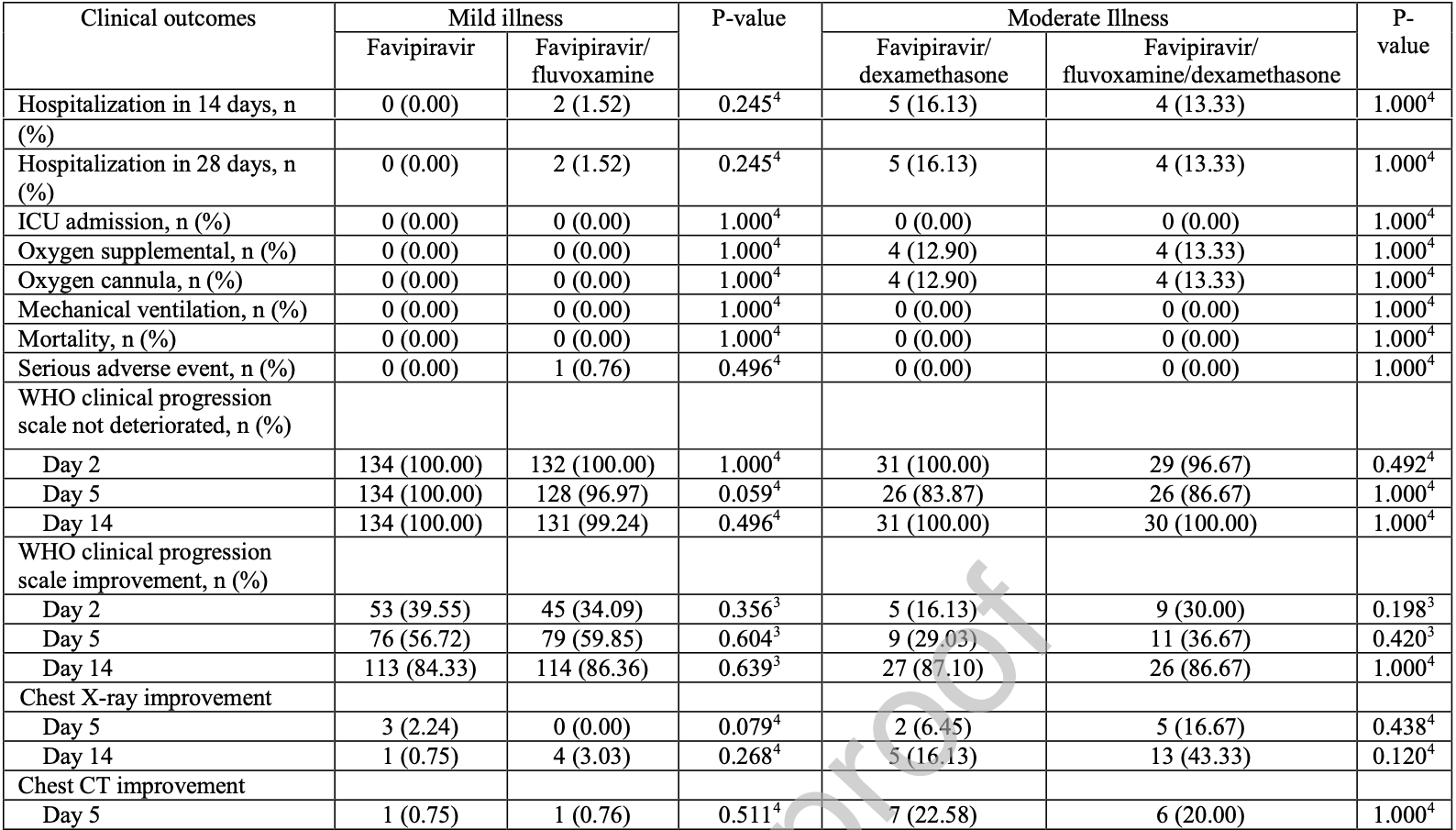
RCT 327 outpatients in Thailand, showing no significant difference with 50mg fluvoxamine bid added to favipiravir. Authors note that trials showing benefit mostly used 100mg bid.
|
risk of oxygen therapy, 1.9% higher, RR 1.02, p = 1.00, treatment 4 of 162 (2.5%), control 4 of 165 (2.4%).
|
|
risk of hospitalization, 22.2% higher, RR 1.22, p = 0.77, treatment 6 of 162 (3.7%), control 5 of 165 (3.0%), day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Siripongboonsitti et al., 29 Jun 2023, Randomized Controlled Trial, Thailand, peer-reviewed, 9 authors, study period 26 June, 2021 - 22 February, 2022, trial TCTR20210615002 (EFFaCo).
Efficacy of Combination Therapy of Fluvoxamine and Favipiravir versus Favipiravir Monotherapy to Prevent Severe COVID-19 among Mild to Moderate COVID-19 Patients: Open-label Randomized Controlled Trial (EFFaCo Study)
doi:10.1016/j.ijid.2023.06.018
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Bast, Tang, Dahn, Palacio, Increased risk of hospitalisation and death with the delta variant in the USA, Lancet Infect Dis, doi:10.1016/s1473-3099
Bernal, Da Silva, Musungaie, Kovalchuk, None
Bramante, Huling, Tignanelli, Buse, Liebovitz et al., Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Chen, Huang, Cheng, Wu, Chen et al., Favipiravir versus arbidol for COVID-19: a randomized clinical trial, MedRxiv, doi:10.1101/2020.03.17
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized, Open-Label Clinical Trial, Clin Infect Dis, doi:10.1093/cid/ciab962
Du, Chen, Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1844
Fung, Liu, The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus, Virology, doi:10.1016/j.virol.2019.05.002
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, doi:10.2183/pjab.93.027
Guo, Harari, Chernecki, Thorlund, Forrest, Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials, Am J Trop Med Hyg, doi:10.4269/ajtmh.21-1310
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Scientific reports, doi:10.1038/s41598-021-90551-6
Hoertel, Sánchez-Rico, De La Muela, Abellán, Blanco et al., Risk of Death in Individuals Hospitalized for COVID-19 With and Without Psychiatric Disorders: An Observational Multicenter Study in France, Biol Psychiatry Glob Open Sci, doi:10.1016/j.bpsgos.2021.12.007
Homolak, Kodvanj, Widely available lysosome targeting agents should be considered as potential therapy for COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106044
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clinical Infectious Diseases, doi:10.1093/cid/ciaa1176
Lee, Vigod, Bortolussi-Courval, Hanula, Boulware et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.6269
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial, Jama, doi:10.1001/jama.2020.22760
Mccarthy, Naggie, Boulware, Lindsell, Stewart et al., Fluvoxamine for Outpatient Treatment of COVID-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial, doi:10.1101/2022.10.17.22281178
Németh, Szûcs, Vitrai, Juhász, Németh et al., Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: A retrospective casecontrol study, Ideggyogy Sz, doi:10.18071/isz.74.0389
Oskotsky, Maric, Tang, Oskotsky, Wong et al., Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.33090
Reis, Santos Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/s2214-109x(21)00448-4
Reis, Santos, Silva, Silva, Thabane et al., Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial, Ann Intern Med, doi:10.7326/m22-3305
Reyes, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Schlienger, Meier, Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?, Am J Cardiovasc Drugs, doi:10.2165/00129784-200303030-00001
Seftel, Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infect Dis, doi:10.1093/ofid/ofab050
Seo, Kim, Bae, Park, Chung et al., Fluvoxamine Treatment of Patients with Symptomatic COVID-19 in a Community Treatment Center: A Preliminary Result of Randomized Controlled Trial, Infect Chemother, doi:10.3947/ic.2021.0142
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virology journal, doi:10.1186/s12985-020-01412-z
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.652688
DOI record:
{
"DOI": "10.1016/j.ijid.2023.06.018",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2023.06.018",
"alternative-id": [
"S1201971223006410"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2023.06.018"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7256-9982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siripongboonsitti",
"given": "Taweegrit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ungtrakul",
"given": "Teerapat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tawinprai",
"given": "Kriangkrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nimmol",
"given": "Tararin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buttakosa",
"given": "Mullika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sornsamdang",
"given": "Gaidganok",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jarrusrojwuttikul",
"given": "Tanadul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silapant",
"given": "Phumin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahanonda",
"given": "Nithi",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
30
]
],
"date-time": "2023-06-30T06:07:12Z",
"timestamp": 1688105232000
},
"deposited": {
"date-parts": [
[
2023,
10,
13
]
],
"date-time": "2023-10-13T19:14:19Z",
"timestamp": 1697224459000
},
"funder": [
{
"DOI": "10.13039/100016175",
"doi-asserted-by": "publisher",
"name": "Chulabhorn Royal Academy"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
26
]
],
"date-time": "2024-03-26T21:51:53Z",
"timestamp": 1711489913784
},
"is-referenced-by-count": 5,
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T00:00:00Z",
"timestamp": 1693526400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
20
]
],
"date-time": "2023-06-20T00:00:00Z",
"timestamp": 1687219200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971223006410?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971223006410?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "211-219",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2023.06.018_bib0001",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2023.06.018_bib0002",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/j.ijid.2023.06.018_bib0003",
"unstructured": "United States Food and Drug Administration, FDA Network of European World Shops. RELEASE, coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19; 2021 [accessed 22 December 2021]."
},
{
"key": "10.1016/j.ijid.2023.06.018_sbref0004",
"series-title": "Clinical practice guideline to diagnosis, treatment and prevention COVID-19 for Physicain and health care provider",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "Martinez-de-Hoyo R. The efficacy and safety of favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"first-page": "11022",
"journal-title": "Sci Rep",
"key": "10.1016/j.ijid.2023.06.018_bib0005",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Virol J",
"key": "10.1016/j.ijid.2023.06.018_bib0006",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab962",
"article-title": "Efficacy of early treatment with favipiravir on disease progression among high-risk patients with coronavirus disease 2019 (COVID-19): a randomized, open-label clinical trial",
"author": "Chuah",
"doi-asserted-by": "crossref",
"first-page": "e432",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2023.06.018_bib0007",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1002/cpt.1844",
"article-title": "Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection",
"author": "Du",
"doi-asserted-by": "crossref",
"first-page": "242",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.ijid.2023.06.018_bib0008",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/j.ijid.2023.06.018_bib0009",
"volume": "93",
"year": "2017"
},
{
"article-title": "Experimental treatment with favipiravir for COVID-19: an open-label control study",
"author": "Cai",
"first-page": "1192",
"journal-title": "Engineering (Beijing)",
"key": "10.1016/j.ijid.2023.06.018_bib0010",
"volume": "6",
"year": "2020"
},
{
"article-title": "Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized clinical trial",
"author": "Chen",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ijid.2023.06.018_bib0011",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a Phase II/III multicenter randomized clinical trial",
"author": "Ivashchenko",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2023.06.018_bib0012",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: a review of its mechanism of action and its role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ijid.2023.06.018_bib0013",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106044",
"article-title": "Widely available lysosome targeting agents should be considered as potential therapy for COVID-19",
"author": "Homolak",
"doi-asserted-by": "crossref",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.ijid.2023.06.018_bib0014",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2019.05.002",
"article-title": "The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "34",
"journal-title": "Virology",
"key": "10.1016/j.ijid.2023.06.018_bib0015",
"volume": "533",
"year": "2019"
},
{
"DOI": "10.2165/00129784-200303030-00001",
"article-title": "Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?",
"author": "Schlienger",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Am J Cardiovasc Drugs",
"key": "10.1016/j.ijid.2023.06.018_bib0016",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2023.06.018_bib0017",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.21-1310",
"article-title": "Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "1315",
"journal-title": "Am J Trop Med Hyg",
"key": "10.1016/j.ijid.2023.06.018_bib0018",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"journal-title": "Lancet Glob Health",
"key": "10.1016/j.ijid.2023.06.018_bib0019",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.7326/M22-3305",
"article-title": "Oral fluvoxamine with inhaled budesonide for treatment of early-onset COVID-19: a randomized platform trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.ijid.2023.06.018_bib0020",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofab050",
"article-title": "Prospective cohort of fluvoxamine for early treatment of coronavirus Disease 19",
"author": "Seftel",
"doi-asserted-by": "crossref",
"first-page": "ofab050",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.ijid.2023.06.018_bib0021",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac492.1881",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ijid.2023.06.018_bib0022",
"unstructured": "McCarthy MW, Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Felker GM, et al. Fluvoxamine for outpatient treatment of COVID-19: a decentralized, placebo-controlled, randomized, platform clinical trial. medRxiv. 01 November 2022. https://www.medrxiv.org/content/10.1101/2022.10.17.22281178 [accessed 2 November 2022]."
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2023.06.018_bib0023",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.18071/isz.74.0389",
"article-title": "Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: a retrospective case-control study",
"author": "Németh",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Ideggyogy Sz",
"key": "10.1016/j.ijid.2023.06.018_bib0024",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1016/j.bpsgos.2021.12.007",
"article-title": "Risk of death in individuals hospitalized for COVID-19 with and without psychiatric disorders: an observational multicenter study in France",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Biol Psychiatry Glob Open Sci",
"key": "10.1016/j.ijid.2023.06.018_bib0025",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"article-title": "Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants",
"author": "Oskotsky",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ijid.2023.06.018_bib0026",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"article-title": "Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ijid.2023.06.018_bib0027",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.3947/ic.2021.0142",
"article-title": "Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial",
"author": "Seo",
"doi-asserted-by": "crossref",
"first-page": "102",
"journal-title": "Infect Chemother",
"key": "10.1016/j.ijid.2023.06.018_bib0028",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00685-X",
"article-title": "Increased risk of hospitalisation and death with the delta variant in the USA",
"author": "Bast",
"doi-asserted-by": "crossref",
"first-page": "1629",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ijid.2023.06.018_bib0029",
"volume": "21",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971223006410"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "134"
}
