Prospective cohort of fluvoxamine for early treatment of COVID-19
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab050, Feb 2021
31st treatment shown to reduce risk in
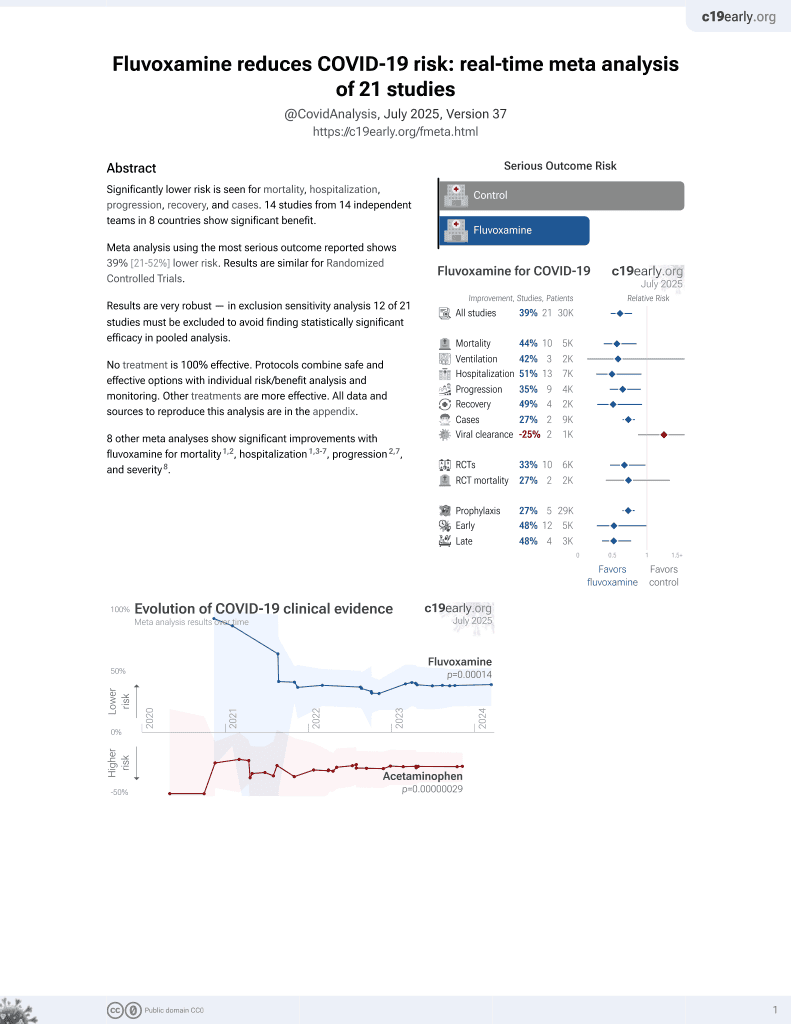
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
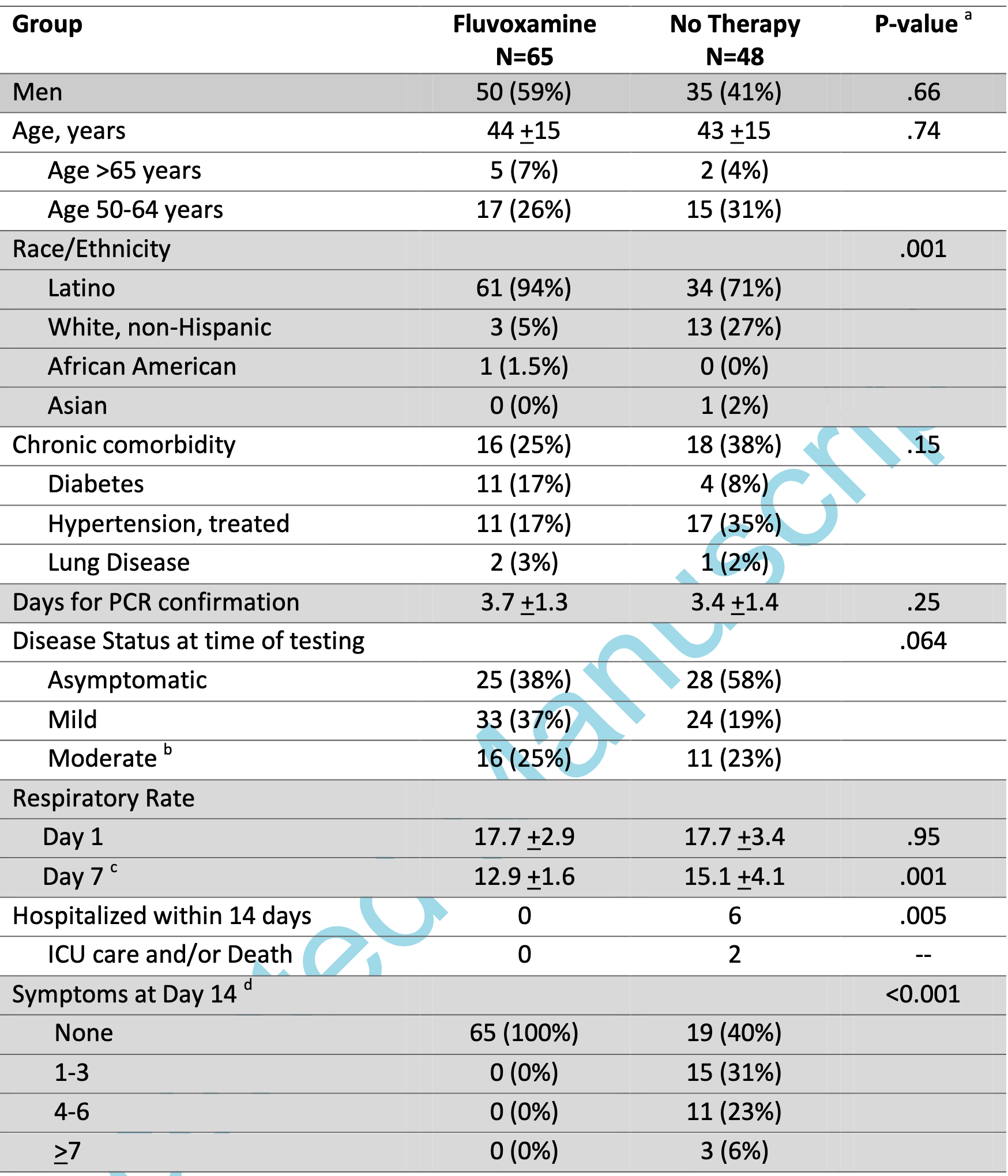
Prospective quasi-randomized (patient choice) study with 125 outpatients, 77 treated with fluvoxamine, showing lower death/ICU admission (0 of 77 vs. 2 of 48), lower hospitalization (0 of 77 vs. 6 of 48), and faster recovery with treatment. Note that 12 treatment patients were added but are not reflected in the table in the paper (because the numbers had been previously published and the IRB did not allow updating the table).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 72.3% lower, RR 0.28, p = 0.38, treatment 0 of 77 (0.0%), control 1 of 48 (2.1%), NNT 48, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of death/ICU, 83.9% lower, RR 0.16, p = 0.15, treatment 0 of 77 (0.0%), control 2 of 48 (4.2%), NNT 24, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 94.0% lower, RR 0.06, p = 0.003, treatment 0 of 77 (0.0%), control 6 of 48 (12.5%), NNT 8.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 98.7% lower, RR 0.01, p < 0.001, treatment 0 of 77 (0.0%), control 29 of 48 (60.4%), NNT 1.7, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Seftel et al., 1 Feb 2021, prospective quasi-randomized (patient choice), USA, peer-reviewed, 2 authors.
Abstract: Open Forum Infectious Diseases
Brief Report
Prospective Cohort of Fluvoxamine
for Early Treatment of Coronavirus
Disease 19
David Seftel1,2 and David R. Boulware3,
1
Golden Gate Fields Medical Clinic, Berkeley, California, USA, 2Enable Biosciences Inc, South
San Francisco, California, USA, 3University of Minnesota Medical School, Minneapolis,
Minnesota, USA
We read with interest Lenze et al’s [1] double-blind randomized
clinical trial testing fluvoxamine for early treatment of coronavirus disease 2019 (COVID-19). In this 152 person outpatient
trial, fluvoxamine decreased clinical progression, defined as hypoxia (<92% oxygen saturation) coupled with either shortness
of breath or hospitalization, from 8% (6 of 72) with observation
alone to 0% (0 of 80) with fluvoxamine at up to 300 mg daily
(P = .009) [1]. Although fluvoxamine is a selective serotonin reuptake inhibitor, fluvoxamine also activates sigma-1 receptors
present intracellularly in the endoplasmic reticulum, thereby
decreasing cytokine production [2]. We wish to share our recent real-world experience using fluvoxamine inspired by this
recent trial.
METHODS
In November–December 2020, a mass outbreak of COVID-19
occurred in an occupational setting with congregate living at
a horse racing track in California. We instituted 3 successive
Received 21 December 2020; editorial decision 26 January 2021; accepted 27 January 2021.
Correspondence: David Seftel, MD, MBA, Medical Director/Primary Care Clinician,
Golden Gate Fields Medical Clinic, 1100 Eastshore Highway, Berkeley, CA 94710 (dseftel@
enablebiosciences.com).
Open Forum Infectious Diseases®2021
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases
Society of America. This is an Open Access article distributed under the terms of the Creative
Commons Attribution-NonCommercial-NoDerivs licence (http://creativecommons.org/licenses/
by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any
medium, provided the original work is not altered or transformed in any way, and that the
work is properly cited. For commercial re-use, please contact journals.permissions@oup.com
DOI: 10.1093/ofid/ofab050
Patient Consent Statement
Patients consented for their medical treatment. As a quality improvement initiative, we assessed 14-day outcomes, analyzing
the existing collected, deidentified data (Institutional Review
Board exempt by the University of Minnesota).
RESULTS
Of 113 SARS-COV-2 antigen positive persons, approximately
half were asymptomatic when initially tested. The median
age was 42 years (interquartile range, 33 to 56), and 75% were
men; 84% were Latino, and 14% were white. In total, 65 persons opted for fluvoxamine, and 48 opted for observation alone
with no therapy. Demographics were generally similar among
those choosing fluvoxamine versus observation, with the exception that more nonwhite persons opted for fluvoxamine (Table
1). Fewer patients opting for fluvoxamine were asymptomatic
(38%) at time of initial diagnostic testing than those opting for
observation (58%). Overall, 30% had 1 or more chronic medical
comorbidities. Those opting for fluvoxamine had slightly more
frequent diabetes (17% vs 8%) and slightly less treated hypertension (17% vs 35%) than those receiving observation.
The incidence of subsequent hospitalization was 0% (0 of
65) with fluvoxamine and 12.5% (6 of 48) with observation
(P = .005). Two persons required intensive care unit..
DOI record:
{
"DOI": "10.1093/ofid/ofab050",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofab050",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>We report a real-world experience using fluvoxamine for coronavirus disease 19 (COVID-19) in a prospective cohort in the setting of a mass outbreak. Overall, 65 persons opted to receive fluvoxamine (50 mg twice daily) and 48 declined. Incidence of hospitalization was 0% (0 of 65) with fluvoxamine and 12.5% (6 of 48) with observation alone. At 14 days, residual symptoms persisted in 0% (0 of 65) with fluvoxamine and 60% (29 of 48) with observation.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Golden Gate Fields Medical Clinic, Berkeley, California, USA"
},
{
"name": "Enable Biosciences Inc, South San Francisco, California, USA"
}
],
"family": "Seftel",
"given": "David",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "University of Minnesota Medical School, Minneapolis, Minnesota, USA"
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T05:16:18Z",
"timestamp": 1612156578000
},
"deposited": {
"date-parts": [
[
2021,
2,
17
]
],
"date-time": "2021-02-17T19:54:14Z",
"timestamp": 1613591654000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T05:19:45Z",
"timestamp": 1712035185717
},
"is-referenced-by-count": 121,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
1
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T00:00:00Z",
"timestamp": 1612137600000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofab050/36152952/ofab050.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/8/2/ofab050/36301401/ofab050.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/ofid/article-pdf/8/2/ofab050/36301401/ofab050.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
2,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
1
]
]
},
"published-other": {
"date-parts": [
[
2021,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
2,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic covid-19: a randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "2021021717275727400_CIT0001",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"article-title": "Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis",
"author": "Rosen",
"doi-asserted-by": "crossref",
"first-page": "eaau5266",
"journal-title": "Sci Transl Med",
"key": "2021021717275727400_CIT0002",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.051718",
"article-title": "Emulating randomized clinical trials with nonrandomized real-world evidence studies: first results from the RCT DUPLICATE Initiative",
"author": "Franklin",
"doi-asserted-by": "crossref",
"journal-title": "Circulation",
"key": "2021021717275727400_CIT0003",
"year": "2020"
}
],
"reference-count": 3,
"references-count": 3,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofab050/6124100"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19",
"type": "journal-article",
"volume": "8"
}