Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.10.010, Oct 2022
30th treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
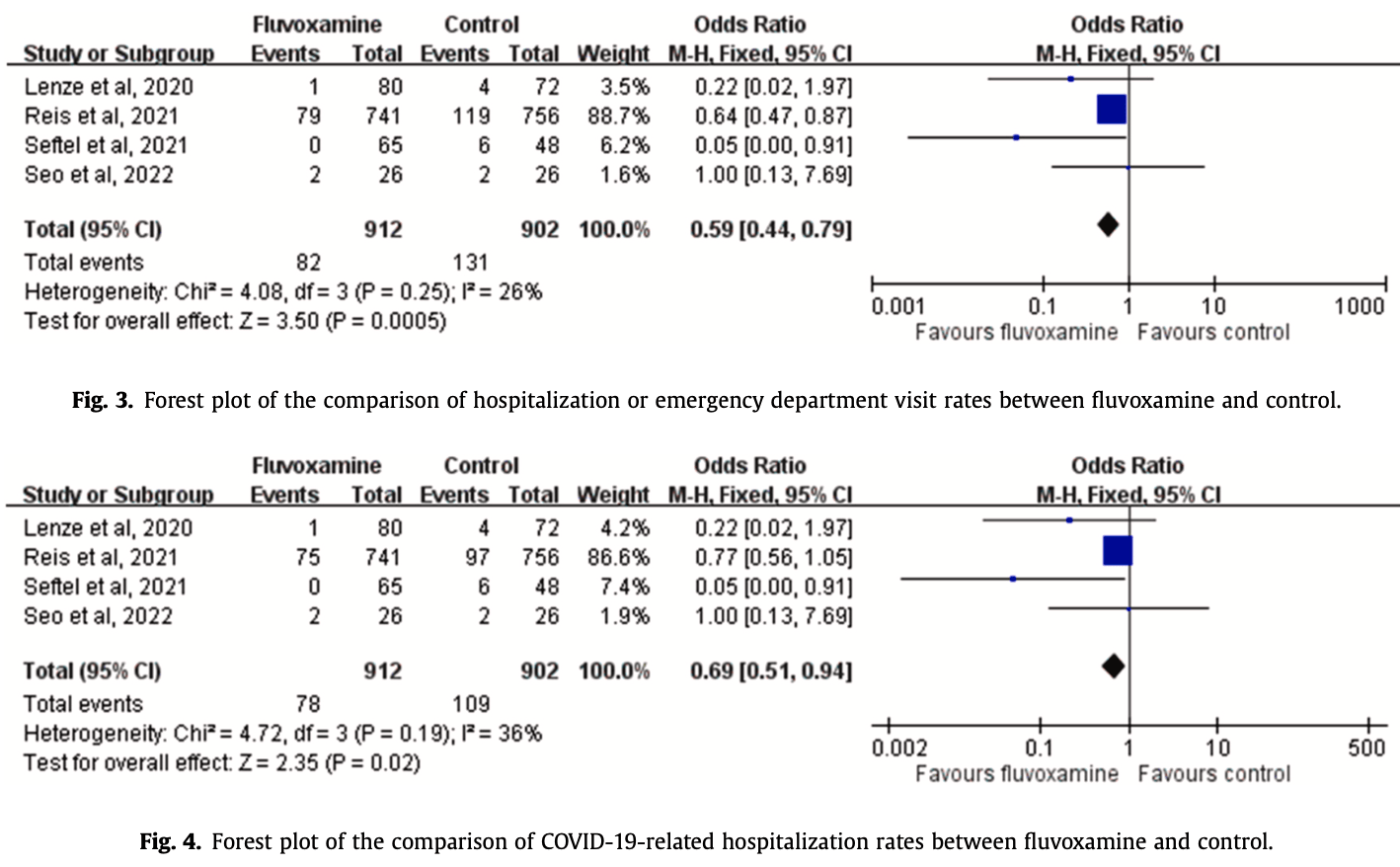
Systematic review and meta analysis of 4 fluvoxamine outpatient trials, showing lower hospitalization with treatment.
9 meta-analyses show significant improvements with fluvoxamine for mortality1-3,
hospitalization1,4-8 ,
progression2,8, and
severity9.
Currently there are 21 fluvoxamine for COVID-19 studies, showing 44% lower mortality [15‑63%], 42% lower ventilation [-151‑86%], 10% higher ICU admission [-72‑326%], 51% lower hospitalization [8‑73%], and 27% fewer cases [18‑35%].
|
risk of hospitalization, 31.0% lower, OR 0.69, p = 0.02, RR approximated with OR.
|
|
risk of hospitalization/ER, 41.0% lower, OR 0.59, p < 0.001, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Deng et al., Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: A systematic review and meta-analysis, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.01.010.
2.
Prasanth et al., A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications, Scientific Reports, doi:10.1038/s41598-024-64260-9.
3.
Fico et al., Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis, European Neuropsychopharmacology, doi:10.1016/j.euroneuro.2022.10.004.
4.
Lee et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.6269.
5.
Lu et al., Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis, Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.10.010.
6.
Marcec et al., A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference, Journal of Infection, doi:10.1016/j.jinf.2022.11.011.
7.
Deng (B) et al., Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis, Reviews in Medical Virology, doi:10.1002/rmv.2501.
Lu et al., 13 Oct 2022, peer-reviewed, 5 authors.
Contact: dtmed141@gmail.com.
Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis
Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.10.010
Objectives: This meta-analysis investigated the use of fluvoxamine for the treatment of nonhospitalized patients with COVID-19. Methods: PubMed, Web of Science, Ovid medline, Embase, Scopus, Cochrane Library databases, and ClinicalTrials.gov were searched for studies published before June 25, 2022. Only clinical studies that compared the efficacy and safety of fluvoxamine with other alternatives or placebos in the treatment of nonhospitalized patients with COVID-19 were included. Results: Four studies with 1814 patients, of whom 912 received fluvoxamine, were included in this study. Compared with the control group receiving placebo or no therapy, the study group receiving fluvoxamine demonstrated a lower risk of hospitalization and emergency department (ED) visits (odds ratio [OR], 0.59; 95 % CI, 0.44-0.79; I 2 = 26 %). In addition, the rate of hospitalization remained significantly lower in patients who received fluvoxamine than in the control group (OR, 0.69; 95 % CI, 0.51-0.94; I 2 = 36 %). Although the study group demonstrated a lower risk of requirement of mechanical ventilation and intensive care unit admission, and mortality than the control group, these differences were nonsignificant. Finally, fluvoxamine use was associated with a similar risk of adverse events as that observed in the control group. Conclusion: Fluvoxamine can be safely used in nonhospitalized patients with COVID-19 and can reduce the hospitalization rate or ED visits in these patients.
Competing interests The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Ethical approval Not required.
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.10.010.
References
Calusic, Marcec, Luksa, Jurkovis, Kovac et al., Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls, Br J Clin Pharm
Chen, Nirula, Heller, Gootlieb, Boscia et al., SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Deeks, Casirivimab/Imdevimab: first approval, Drugs
Dougan, Nirula, Azizad, Mocherla, Gootlieb et al., Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med
Gandhi, Lynch, Rio, Mild or moderate Covid-19, N Engl J Med
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Guo, Harari, Chernecki, Thorlund, Forrest, Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials, Am J Trop Med Hyg
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Hashimoto, Suzuki, Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: a historical review, Mol Psychiatry
Hashimoto, Suzuki, Hashimoto, Old drug fluvoxamine, new hope for COVID-19, Eur Arch Psychiatry Clin Neurosci
Hoertel, Sánchez-Rico, Vernet, Beeker, Jannot et al., Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry
Kornhuber, Hoertel, Gulbins, The acid sphingomyelinase/ceramide system in COVID-19, Mol Psychiatry
Lai, Liu, Wang, Wang, Hsueh et al., Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths, J Microbiol Immunol Infect
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges, Int J Antimicrob Agents
Lee, Vigod, Bortolussi-Courval, Hanula, Boulware et al., Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis, JAMA Netw Open
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Mahase, Covid-19: Molnupiravir reduces risk of hospital admission or death by 50 % in patients at risk, MSD reports, BMJ
Moher, Liberati, Tetzlaff, Altman, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, BMJ
Mueller, Riederer, Müller, Neuropsychiatric drugs against COVID-19: What is the clinical evidence?, Pharmacopsychiatry
Reis, Santos Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health
Seftel, Boulware, Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19, Open Forum Infect Dis
Seo, Kim, Bae, Park, Chung et al., Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial, Infect Chemother
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: a review of its mechanism of action and its role in COVID-19, Front Pharm
DOI record:
{
"DOI": "10.1016/j.jiph.2022.10.010",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2022.10.010",
"alternative-id": [
"S1876034122002659"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2022.10.010"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Lu",
"given": "Li-Chin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Chao",
"given": "Chien-Ming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Shen-Peng",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8663-3161",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lan",
"given": "Shao-Huan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6334-2388",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lai",
"given": "Chih-Cheng",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
13
]
],
"date-time": "2022-10-13T11:02:38Z",
"timestamp": 1665658958000
},
"deposited": {
"date-parts": [
[
2022,
10,
20
]
],
"date-time": "2022-10-20T01:11:47Z",
"timestamp": 1666228307000
},
"indexed": {
"date-parts": [
[
2022,
10,
20
]
],
"date-time": "2022-10-20T05:03:26Z",
"timestamp": 1666242206239
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2022,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T00:00:00Z",
"timestamp": 1667260800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
9
]
],
"date-time": "2022-10-09T00:00:00Z",
"timestamp": 1665273600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034122002659?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034122002659?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1259-1264",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jiph.2022.10.010_bib1",
"unstructured": "World Health Organization. https://covid19.who.int/ Accessed on July 1, 2021."
},
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"article-title": "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges",
"author": "Lai",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.jiph.2022.10.010_bib2",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009249",
"article-title": "Mild or moderate Covid-19",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1757",
"issue": "18",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.10.010_bib3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.jmii.2020.02.012",
"article-title": "Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "404",
"issue": "3",
"journal-title": "J Microbiol Immunol Infect",
"key": "10.1016/j.jiph.2022.10.010_bib4",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.10.010_bib5",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.10.010_bib6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2022.10.010_bib7",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.10.010_bib8",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01021-4",
"article-title": "Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "5199",
"issue": "9",
"journal-title": "Mol Psychiatry",
"key": "10.1016/j.jiph.2022.10.010_bib9",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: a review of its mechanism of action and its role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharm",
"key": "10.1016/j.jiph.2022.10.010_bib10",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01309-5",
"article-title": "The acid sphingomyelinase/ceramide system in COVID-19",
"author": "Kornhuber",
"doi-asserted-by": "crossref",
"first-page": "307",
"issue": "1",
"journal-title": "Mol Psychiatry",
"key": "10.1016/j.jiph.2022.10.010_bib11",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41380-021-01432-3",
"article-title": "Mechanisms of action of fluvoxamine for COVID-19: a historical review",
"author": "Hashimoto",
"doi-asserted-by": "crossref",
"first-page": "1898",
"issue": "4",
"journal-title": "Mol Psychiatry",
"key": "10.1016/j.jiph.2022.10.010_bib12",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1055/a-1717-2381",
"article-title": "Neuropsychiatric drugs against COVID-19: What is the clinical evidence?",
"author": "Mueller",
"doi-asserted-by": "crossref",
"first-page": "7",
"issue": "1",
"journal-title": "Pharmacopsychiatry",
"key": "10.1016/j.jiph.2022.10.010_bib13",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1007/s00406-021-01326-z",
"article-title": "Old drug fluvoxamine, new hope for COVID-19",
"author": "Hashimoto",
"doi-asserted-by": "crossref",
"first-page": "161",
"issue": "1",
"journal-title": "Eur Arch Psychiatry Clin Neurosci",
"key": "10.1016/j.jiph.2022.10.010_bib14",
"volume": "272",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"issue": "22",
"journal-title": "JAMA",
"key": "10.1016/j.jiph.2022.10.010_bib15",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1093/ofid/ofab050",
"article-title": "Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19",
"author": "Seftel",
"doi-asserted-by": "crossref",
"first-page": "ofab050",
"issue": "2",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.jiph.2022.10.010_bib16",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"issue": "1",
"journal-title": "Lancet Glob Health",
"key": "10.1016/j.jiph.2022.10.010_bib17",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1136/bmj.b2535",
"article-title": "Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement",
"author": "Moher",
"doi-asserted-by": "crossref",
"first-page": "b2535",
"journal-title": "BMJ",
"key": "10.1016/j.jiph.2022.10.010_bib18",
"volume": "339",
"year": "2009"
},
{
"DOI": "10.1136/bmj.l4898",
"article-title": "RoB 2: a revised tool for assessing risk of bias in randomised trials",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "l4898",
"journal-title": "BMJ",
"key": "10.1016/j.jiph.2022.10.010_bib19",
"volume": "366",
"year": "2019"
},
{
"DOI": "10.3947/ic.2021.0142",
"article-title": "Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial",
"author": "Seo",
"doi-asserted-by": "crossref",
"first-page": "102",
"issue": "1",
"journal-title": "Infect Chemother",
"key": "10.1016/j.jiph.2022.10.010_bib20",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.4269/ajtmh.21-1310",
"article-title": "Fluvoxamine for the early treatment of COVID-19: a meta-analysis of randomized clinical trials",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "1315",
"issue": "5",
"journal-title": "Am J Trop Med Hyg",
"key": "10.1016/j.jiph.2022.10.010_bib21",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"article-title": "Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2022.10.010_bib22",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1111/bcp.15126",
"article-title": "Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls",
"author": "Calusic",
"doi-asserted-by": "crossref",
"first-page": "2065",
"issue": "5",
"journal-title": "Br J Clin Pharm",
"key": "10.1016/j.jiph.2022.10.010_bib23",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1007/s40265-021-01620-z",
"article-title": "Casirivimab/Imdevimab: first approval",
"author": "Deeks",
"doi-asserted-by": "crossref",
"first-page": "2047",
"issue": "17",
"journal-title": "Drugs",
"key": "10.1016/j.jiph.2022.10.010_bib24",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2422",
"article-title": "Covid-19: Molnupiravir reduces risk of hospital admission or death by 50 % in patients at risk, MSD reports",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2422",
"journal-title": "BMJ",
"key": "10.1016/j.jiph.2022.10.010_bib25",
"volume": "375",
"year": "2021"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034122002659"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "15"
}
