Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis
et al., Reviews in Medical Virology, doi:10.1002/rmv.2501, Dec 2023
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
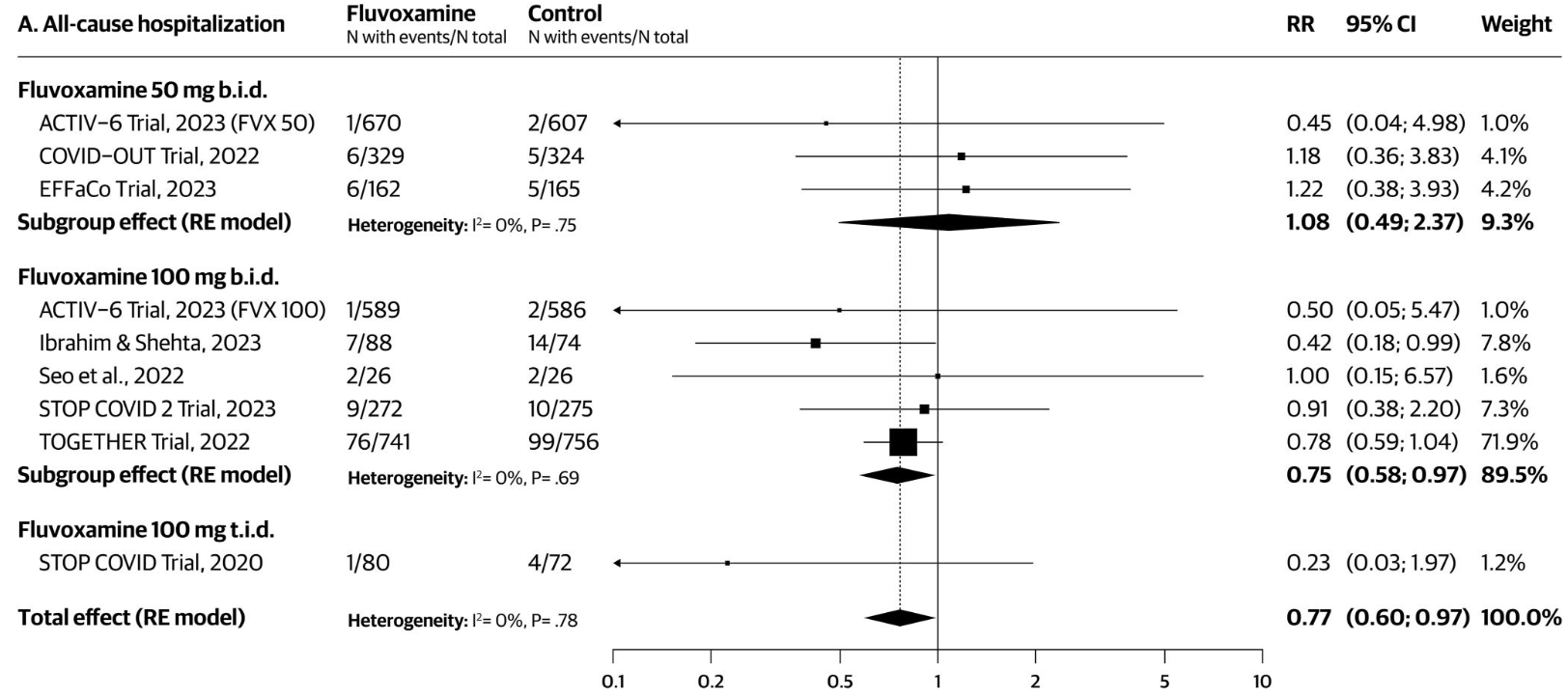
Systematic review and meta-analysis of 9 RCTs (N=5,861) evaluating fluvoxamine for the outpatient treatment of COVID-19, showing lower hospitalization with treatment, with improved results for higher doses. 100mg BID/TID showed lower hospitalization, while 50mg BID did not.
9 meta-analyses show significant improvements with fluvoxamine for mortality1-3,
hospitalization1,4-8 ,
progression2,8, and
severity9.
Currently there are 21 fluvoxamine for COVID-19 studies, showing 44% lower mortality [15‑63%], 42% lower ventilation [-151‑86%], 10% higher ICU admission [-72‑326%], 51% lower hospitalization [8‑73%], and 27% fewer cases [18‑35%].
|
risk of hospitalization, 23.0% lower, RR 0.77, p = 0.03.
|
|
risk of hospitalization, 77.0% lower, RR 0.23, p = 0.17, 100mg TID.
|
|
risk of hospitalization, 25.0% lower, RR 0.75, p = 0.03, 100mg BID.
|
|
risk of hospitalization, 8.0% higher, RR 1.08, p = 0.86, 50mg BID.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Deng et al., Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: A systematic review and meta-analysis, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2023.01.010.
2.
Prasanth et al., A systematic review and meta-analysis, investigating dose and time of fluvoxamine treatment efficacy for COVID-19 clinical deterioration, death, and Long-COVID complications, Scientific Reports, doi:10.1038/s41598-024-64260-9.
3.
Fico et al., Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis, European Neuropsychopharmacology, doi:10.1016/j.euroneuro.2022.10.004.
4.
Lee et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.6269.
5.
Lu et al., Effect of fluvoxamine on outcomes of nonhospitalized patients with COVID-19: A systematic review and meta-analysis, Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.10.010.
6.
Marcec et al., A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference, Journal of Infection, doi:10.1016/j.jinf.2022.11.011.
7.
Deng (B) et al., Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis, Reviews in Medical Virology, doi:10.1002/rmv.2501.
Deng et al., 26 Dec 2023, peer-reviewed, 11 authors.
Contact: dengj35@mcmaster.ca.
Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis
Reviews in Medical Virology, doi:10.1002/rmv.2501
This systematic review and meta-analysis of randomised controlled trials (RCTs) aimed to evaluate the efficacy, safety, and tolerability of fluvoxamine for the outpatient management of COVID-19. We conducted this review in accordance with the PRISMA 2020 guidelines. Literature searches were conducted in MED-LINE, EMBASE, International Pharmaceutical Abstracts, CINAHL, Web of Science, and CENTRAL up to 14 September 2023. Outcomes included incidence of hospitalisation, healthcare utilization (emergency room visits and/or hospitalisation), mortality, supplemental oxygen and mechanical ventilation requirements, serious adverse events (SAEs) and non-adherence. Fluvoxamine 100 mg twice a day was associated with reductions in the risk of hospitalisation (risk ratio [RR] 0.75, 95% confidence interval [CI] 0.58-0.97; I 2 = 0%) and reductions in the risk of healthcare utilization (RR 0.68, 95% CI 0.53-0.86; I 2 = 0%). While no increased SAEs were observed, fluvoxamine 100 mg twice a day was associated with higher treatment non-adherence compared to placebo (RR 1.61, 95% CI 1.22-2.14; I 2 = 53%). In subgroup analyses, fluvoxamine reduced healthcare utilization in outpatients with BMI ≥30 kg/m 2 , but not in those with lower BMIs. While fluvoxamine offers potential benefits in reducing healthcare utilization, its efficacy may be most pronounced in high-risk patient populations. The observed non-adherence rates highlight the need for better patient education and counselling. Future investigations should reassess trial endpoints to include outcomes relating to post-COVID sequelaes. Registration: This review was prospectively registered on PROSPERO (CRD42023463829).
| Study limitations The major limitation of this study is the potential presence of smallstudy effects for the outcome of hospitalisation and the requirement Note: GRADE Working Group quality of evidence rating. 28 High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. Abbreviations: CI, Confidence Interval; RR, Risk Ratio. a The absolute risk in the intervention group (and its 95% confidence interval) is based on the baseline risk in the control group and the relative effect of the intervention (and its 95% CI). The baseline risk in the control group is calculated using GRADEpro GDT, by dividing the total control group sample size by the total incidence in the control group. This is different from the methods used for NNT calculations, which is the average control risk in the included studies for each outcome. b Downgraded by one level due to limitations in study design or execution (risk of bias); one of the included studies..
References
Anderson, Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability, J Affect Disord, doi:10.1016/s0165-0327(99)00092-0
Bhimraj, Gallagher, Lack of benefit of fluvoxamine for COVID-19 [internet, JAMA, doi:10.1001/jama.2022.23954
Boldrini, Canoll, Klein, How COVID-19 affects the brain, JAMA Psychiatry, doi:10.1001/jamapsychiatry.2021.0500
Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med [Internet, doi:10.1056/NEJMoa2201662
Bull, Hunkeler, Lee, Discontinuing or switching selective serotonin-reuptake inhibitors, Ann Pharmacother, doi:10.1345/aph.1A254
Cook, Sackett, The number needed to treat: a clinically useful measure of treatment effect, BMJ [Internet, doi:10.1136/bmj.310.6977.452
Deng, Heybati, Ramaraju, Zhou, Rayner et al., Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis, Infection [Internet, doi:10.1007/s15010-022-01825-8
Deng, None
Deng, Rayner, Ramaraju, Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2023.01.010
Dessie, Zewotir, Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients, BMC Infect Dis, doi:10.1186/s12879-021-06536-3
Flaherty, A simple method for evaluating the clinical literature, Fam Pract Manag
Greenblatt, Gupta, Kao, Drug repurposing during the COVID-19 pandemic: lessons for expediting drug development and access, Health Aff [Internet, doi:10.1377/hlthaff.2022.01083
Guyatt, Oxman, Montori, GRADE guidelines: 5. Rating the quality of evidence--publication bias, J Clin Epidemiol, doi:10.1016/j.jclinepi.2011.01.011
Guyatt, Oxman, Santesso, GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes, J Clin Epidemiol, doi:10.1016/j.jclinepi.2012.01.012
Guyatt, Oxman, Vist, GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, BMJ [Internet, doi:10.1136/bmj.39489.470347
Harbord, Egger, Sterne, A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints, Stat Med [Internet, doi:10.1002/sim.2380
Hashimoto, Overview of the potential use of fluvoxamine for COVID-19 and long COVID, Discov Ment Health, doi:10.1007/s44192-023-00036-3
Higgins, Thomas, Chandler, Cochrane Handbook for Systematic Reviews of Interventions
Ibrahim, Shehta, Treatment with fluvoxamine in nonhospitalized coronavirus disease 2019 patients, J Chest Dis Tuberc, doi:10.4103/ecdt.ecdt_38_22
Irons, Fluvoxamine in the treatment of anxiety disorders, Neuropsychiatr Dis Treat
Keyloun, Hansen, Hepp, Gillard, Thase et al., Adherence and persistence across antidepressant therapeutic Classes: a retrospective Claims analysis among insured US patients with major depressive disorder (MDD), CNS Drugs, doi:10.1007/s40263-017-0417-0
Lee, Murthy, Corpo, Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.04.018
Lenze, Mattar, Zorumski, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA [Internet, doi:10.1001/jama.2020.22760
Mccarthy, Naggie, Boulware, Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, JAMA [Internet, doi:10.1001/jama.2022.24100
Naggie, Effect of higher-dose fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial, medRxiv, doi:10.1101/2023.09.12.23295424
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ [Internet, doi:10.1136/bmj.n71
Reiersen, Mattar, Ignacio, The STOP COVID 2 study: fluvoxamine vs placebo for outpatients with symptomatic COVID-19, a fully remote randomized controlled trial, Open Forum Infect Dis, doi:10.1093/ofid/ofad419
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health [Internet, doi:10.1016/s2214-109x(21)00448-4
Reis, Silva, Silva, Oral fluvoxamine with inhaled budesonide for treatment of early-onset COVID-19, Ann Intern Med [Internet, doi:10.7326/m22-3305
Sansone, Sansone, Antidepressant adherence: are patients taking their medications?, Innov Clin Neurosci
Schwarzer, Carpenter, Rucker, Meta-Analysis with R [Internet, doi:10.1007/978-3-319-21416-0
Seo, Kim, Bae, Fluvoxamine treatment of patients with symptomatic COVID-19 in a Community treatment Center: a preliminary result of randomized controlled trial, Infect Chemother [Internet, doi:10.3947/ic.2021.0142
Siripongboonsitti, Ungtrakul, Tawinprai, Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe among mild to moderate COVID-19 patients: open-label randomized controlled trial (EFFaCo study), Int J Infect Dis [Internet, doi:10.1016/j.ijid.2023.06.018
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: a review of its mechanism of action and its role in COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.652688
Sweeting, Sutton, Lambert, What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data, Stat Med [Internet, doi:10.1002/sim.1761
Tan, Liu, Zhou, Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China, Immunology, doi:10.1111/imm.13223
Terrin, Schmid, Lau, In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias, J Clin Epidemiol, doi:10.1016/j.jclinepi.2005.01.006
Wang, Liu, Zhang, Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: a world-wide review, J Med Virol, doi:10.1002/jmv.28118
Wolff, Nee, Hickey, Marschollek, Risk factors for Covid-19 severity and fatality: a structured literature review, Infection [Internet, doi:10.1007/s15010-020-01509-1
Zeng, Brignardello-Petersen, Hultcrantz, GRADE Guidance 34: update on rating imprecision using a minimally contextualized approach, J Clin Epidemiol [Internet, doi:10.1016/j.jclinepi.2022.07.014
Zhou, Deng, Heybati, Efficacy and safety of corticosteroid regimens for the treatment of hospitalized COVID-19 patients: a meta-analysis, Future Virol, doi:10.2217/fvl-2021-0244
DOI record:
{
"DOI": "10.1002/rmv.2501",
"ISSN": [
"1052-9276",
"1099-1654"
],
"URL": "http://dx.doi.org/10.1002/rmv.2501",
"abstract": "<jats:title>Abstract</jats:title><jats:p>This systematic review and meta‐analysis of randomised controlled trials (RCTs) aimed to evaluate the efficacy, safety, and tolerability of fluvoxamine for the outpatient management of COVID‐19. We conducted this review in accordance with the PRISMA 2020 guidelines. Literature searches were conducted in MEDLINE, EMBASE, International Pharmaceutical Abstracts, CINAHL, Web of Science, and CENTRAL up to 14 September 2023. Outcomes included incidence of hospitalisation, healthcare utilization (emergency room visits and/or hospitalisation), mortality, supplemental oxygen and mechanical ventilation requirements, serious adverse events (SAEs) and non‐adherence. Fluvoxamine 100 mg twice a day was associated with reductions in the risk of hospitalisation (risk ratio [RR] 0.75, 95% confidence interval [CI] 0.58–0.97; <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 0%) and reductions in the risk of healthcare utilization (RR 0.68, 95% CI 0.53–0.86; <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 0%). While no increased SAEs were observed, fluvoxamine 100 mg twice a day was associated with higher treatment non‐adherence compared to placebo (RR 1.61, 95% CI 1.22–2.14; <jats:italic>I</jats:italic><jats:sup>2</jats:sup> = 53%). In subgroup analyses, fluvoxamine reduced healthcare utilization in outpatients with BMI ≥30 kg/m<jats:sup>2</jats:sup>, but not in those with lower BMIs. While fluvoxamine offers potential benefits in reducing healthcare utilization, its efficacy may be most pronounced in high‐risk patient populations. The observed non‐adherence rates highlight the need for better patient education and counselling. Future investigations should reassess trial endpoints to include outcomes relating to post‐COVID sequelaes. Registration: This review was prospectively registered on PROSPERO (CRD42023463829).</jats:p>",
"alternative-id": [
"10.1002/rmv.2501"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-10-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-12-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-12-26"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8274-6468",
"affiliation": [
{
"name": "Temerty Faculty of Medicine University of Toronto Toronto Ontario Canada"
},
{
"name": "Li Ka Shing Knowledge Institute St. Michael's Hospital Toronto Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Deng",
"given": "Jiawen",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0003-4136-6981",
"affiliation": [
{
"name": "Biostatistics Division Dalla Lana School of Public Health University of Toronto Toronto Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Moskalyk",
"given": "Myron",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8491-4090",
"affiliation": [
{
"name": "UBC Faculty of Medicine University of British Columbia Vancouver British Columbia Canada"
}
],
"authenticated-orcid": false,
"family": "Zuo",
"given": "Qi Kang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0564-8776",
"affiliation": [
{
"name": "Temerty Faculty of Medicine University of Toronto Toronto Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Garcia",
"given": "Cristian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9487-8249",
"affiliation": [
{
"name": "Schulich School of Medicine & Dentistry (Windsor) Western University Windsor Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Abbas",
"given": "Umaima",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5148-658X",
"affiliation": [
{
"name": "VCU School of Medicine Virginia Commonwealth University Richmond Virginia USA"
}
],
"authenticated-orcid": false,
"family": "Ramaraju",
"given": "Harikrishnaa Ba",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6699-3090",
"affiliation": [
{
"name": "Department of Health Research Methods, Evidence, and Impact Faculty of Health Sciences McMaster University Hamilton Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Rayner",
"given": "Daniel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0008-1068-8992",
"affiliation": [
{
"name": "Temerty Faculty of Medicine University of Toronto Toronto Ontario Canada"
},
{
"name": "Li Ka Shing Knowledge Institute St. Michael's Hospital Toronto Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Park",
"given": "Ye‐Jean",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4465-2249",
"affiliation": [
{
"name": "Mayo Clinic Alix School of Medicine (Jacksonville) Mayo Clinic Jacksonville Florida USA"
}
],
"authenticated-orcid": false,
"family": "Heybati",
"given": "Kiyan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6236-764X",
"affiliation": [
{
"name": "Faculty of Health Sciences McMaster University Hamilton Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Zhou",
"given": "Fangwen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2382-8631",
"affiliation": [
{
"name": "Temerty Faculty of Medicine University of Toronto Toronto Ontario Canada"
}
],
"authenticated-orcid": false,
"family": "Lohit",
"given": "Simran",
"sequence": "additional"
}
],
"container-title": "Reviews in Medical Virology",
"container-title-short": "Reviews in Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T01:19:05Z",
"timestamp": 1703639945000
},
"deposited": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T01:19:10Z",
"timestamp": 1703639950000
},
"indexed": {
"date-parts": [
[
2023,
12,
27
]
],
"date-time": "2023-12-27T05:06:47Z",
"timestamp": 1703653607469
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
26
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
26
]
],
"date-time": "2023-12-26T00:00:00Z",
"timestamp": 1703548800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/rmv.2501",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
12,
26
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
26
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1377/hlthaff.2022.01083",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.1016/j.cmi.2022.04.018",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.2217/fvl‐2021‐0244",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1007/s15010‐020‐01509‐1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.3389/fphar.2021.652688",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1111/imm.13223",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.1016/s2214‐109x(21)00448‐4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1001/jama.2020.22760",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1056/NEJMoa2201662",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1001/jama.2022.24100",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1016/j.cmi.2023.01.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"article-title": "Antidepressant adherence: are patients taking their medications?",
"author": "Sansone RA",
"first-page": "41",
"journal-title": "Innov Clin Neurosci [Internet]",
"key": "e_1_2_11_13_1",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1007/s40263‐017‐0417‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1101/2023.09.12.23295424",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.4103/ecdt.ecdt_38_22",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1016/j.ijid.2023.06.018",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1093/ofid/ofad419",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1136/bmj.n71",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1002/9781119536604",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1007/s15010‐022‐01825‐8",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"article-title": "Fluvoxamine in the treatment of anxiety disorders",
"author": "Irons J",
"first-page": "289",
"journal-title": "Neuropsychiatr Dis Treat [Internet]",
"key": "e_1_2_11_22_1",
"volume": "1",
"year": "2005"
},
{
"DOI": "10.1007/978-3-319-21416-0",
"author": "Schwarzer G",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_23_1",
"volume-title": "Meta‐Analysis with R [Internet]",
"year": "2015"
},
{
"DOI": "10.1002/sim.1761",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1136/bmj.310.6977.452",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1002/sim.2380",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.1016/j.jclinepi.2011.01.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"DOI": "10.1186/s12879‐021‐06536‐3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.1136/bmj.39489.470347",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1016/j.jclinepi.2022.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1016/j.jclinepi.2012.01.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.3947/ic.2021.0142",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"article-title": "A simple method for evaluating the clinical literature",
"author": "Flaherty RJ",
"first-page": "47",
"journal-title": "Fam Pract Manag",
"key": "e_1_2_11_33_1",
"volume": "11",
"year": "2004"
},
{
"DOI": "10.1002/jmv.28118",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"DOI": "10.1016/s0165‐0327(99)00092‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.1345/aph.1A254",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"DOI": "10.1001/jamapsychiatry.2021.0500",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_37_1"
},
{
"DOI": "10.1007/s44192‐023‐00036‐3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1001/jama.2022.23954",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.7326/m22‐3305",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.1016/j.jclinepi.2005.01.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/rmv.2501"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Evaluating fluvoxamine for the outpatient treatment of COVID‐19: A systematic review and meta‐analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
