The STOP COVID 2 study: Fluvoxamine vs placebo for outpatients with symptomatic COVID-19, a fully-remote randomized controlled trial
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad419 (results 8/20/2021), STOP COVID 2, NCT04668950, Aug 2021
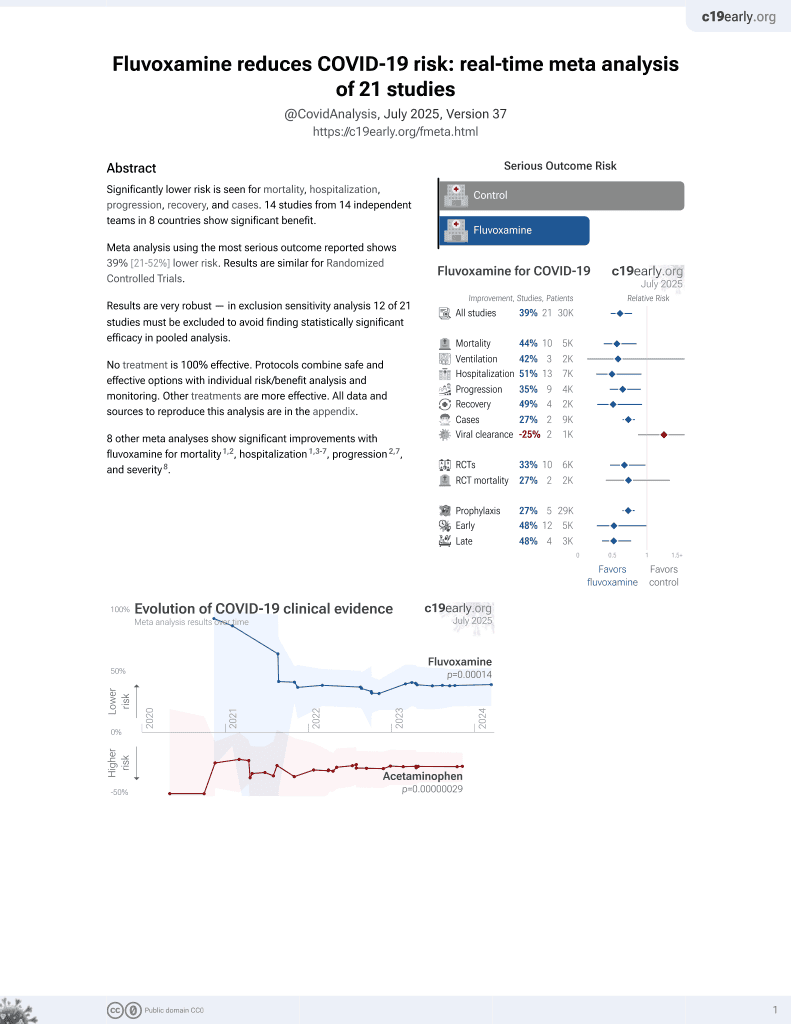
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Remote RCT 547 outpatients a median of 5 days from onset, showing no significant differences with fluvoxamine. The trial was stopped early and underpowered due to low event rates. The trial does not report outcomes that may not be underpowered like time to recovery. Authors note that treatment may have been too late.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of oxygen therapy, 201.1% higher, RR 3.01, p = 0.50, treatment 1 of 272 (0.4%), control 0 of 275 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), non-invasive ventilation.
|
|
risk of oxygen therapy, 32.6% lower, RR 0.67, p = 0.60, treatment 6 of 272 (2.2%), control 9 of 275 (3.3%), NNT 94.
|
|
risk of oxygen therapy, 37.5% lower, RR 0.62, p = 0.74, treatment 3 of 164 (1.8%), control 6 of 205 (2.9%), NNT 91, per-protocol.
|
|
risk of hospitalization, 9.0% lower, RR 0.91, p = 1.00, treatment 9 of 272 (3.3%), control 10 of 275 (3.6%), NNT 305.
|
|
risk of progression, 12.4% lower, RR 0.88, p = 0.85, treatment 13 of 272 (4.8%), control 15 of 275 (5.5%), NNT 148, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Reiersen et al., 20 Aug 2021, Double Blind Randomized Controlled Trial, USA, peer-reviewed, median age 47.0 (treatment) 48.0 (control), 24 authors, study period 22 December, 2020 - 21 May, 2021, average treatment delay 5.0 days, trial NCT04668950 (history) (STOP COVID 2).
The STOP COVID 2 study: Fluvoxamine vs placebo for outpatients with symptomatic COVID-19, a fully-remote randomized controlled trial
Open Forum Infectious Diseases, doi:10.1093/ofid/ofad419
Background: Prior randomized clinical trials have reported benefit of fluvoxamine >200mg/day vs placebo for patients infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Methods: This randomized, double-blind, placebo-controlled, fully-remote multi-site clinical trial evaluated whether fluvoxamine prevents clinical deterioration in higher-risk outpatients with acute COVID-19. Between December 2020 and May 2021, non-hospitalized US and Canadian participants with confirmed symptomatic infection received fluvoxamine (50mg on Day 1, 100mg twice daily thereafter) or placebo for 15 days. The primary modified intent -to-treat (mITT) population included participants who started the intervention within 7 days of symptom onset with a baseline oxygen saturation ≥ 92%. The primary outcome was clinical deterioration within 15 days of randomization defined as having both (1) shortness of breath (severity ≥ 4 on 0-10 scale or requiring hospitalization), and (2) oxygen saturation <92% on room air or need for supplemental oxygen. Results: A total of 547 participants were randomized and met mITT criteria (n=272 fluvoxamine, n=275 placebo). The Data Safety Monitoring Board recommended stopping early for futility related to lower than predicted event rate and declining accrual concurrent with vaccine availability in the U.S. and Canada. Clinical deterioration occurred in 13 (4.8%) participants in the fluvoxamine group and 15 (5.5%) participants in the placebo group (absolute difference at day 15, 0.68% [95% CI, -3.0 % to 4.4 %]; log rank P=0.91). Conclusions: This trial did not find fluvoxamine efficacious in preventing clinical deterioration in unvaccinated outpatients with symptomatic COVID-19. It was stopped early and underpowered due to low primary outcome rates.
References
Bramante, Huling, Tignanelli, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Cummings, Clinical Trials Without Clinical Sites, JAMA Intern Med, doi:10.1001/jamainternmed.2020.9223
Gottlieb, Vaca, Paredes, Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Ibrahim, Lowe, Bramante, Metformin and Covid-19: Focused Review of Mechanisms and Current Literature Suggesting Benefit, Front Endocrinol (Lausanne), doi:10.3389/fendo.2021.587801
Kornhuber, Hoertel, Gulbins, The acid sphingomyelinase/ceramide system in COVID -19. Mol Psychiatry, doi:10.1038/s41380-021-01309-5
Lee, Vigod, Bortolussi-Courval, Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.6269
Lenze, Mattar, Zorumski, Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.22760
Mccarthy, Naggie, Boulware, Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.24100
Reis, Santos Moreira-Silva, Silva, Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID -19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/S2214-109X(21)00448-4
Reis, Santos, Silva, Silva, Oral Fluvoxamine With Inhaled Budesonide for Treatment of Early-Onset COVID-19 : A Randomized Platform Trial, Ann Intern Med, doi:10.7326/M22-3305
Rosen, Seki, Fernandez-Castaneda, Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis, Sci Transl Med. Feb, doi:10.1126/scitranslmed.aau5266
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19. Perspective, Frontiers in Pharmacology, doi:10.3389/fphar.2021.652688
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Zaid, Guessous, Puhm, Platelet reactivity to thrombin differs between patients with COVID-19 and those with ARDS unrelated to COVID-19, Blood Adv, doi:10.1182/bloodadvances.2020003513
DOI record:
{
"DOI": "10.1093/ofid/ofad419",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofad419",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Prior randomized clinical trials have reported benefit of fluvoxamine ≥200 mg/d vs placebo for patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This randomized, double-blind, placebo-controlled, fully remote multisite clinical trial evaluated whether fluvoxamine prevents clinical deterioration in higher-risk outpatients with acute coronavirus disease 2019 (COVID-19). Between December 2020 and May 2021, nonhospitalized US and Canadian participants with confirmed symptomatic infection received fluvoxamine (50 mg on day 1, 100 mg twice daily thereafter) or placebo for 15 days. The primary modified intent-to-treat (mITT) population included participants who started the intervention within 7 days of symptom onset with a baseline oxygen saturation ≥92%. The primary outcome was clinical deterioration within 15 days of randomization, defined as having both (1) shortness of breath (severity ≥4 on a 0–10 scale or requiring hospitalization) and (2) oxygen saturation &lt;92% on room air or need for supplemental oxygen.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 547 participants were randomized and met mITT criteria (n = 272 fluvoxamine, n = 275 placebo). The Data Safety Monitoring Board recommended stopping early for futility related to lower-than-predicted event rates and declining accrual concurrent with vaccine availability in the United States and Canada. Clinical deterioration occurred in 13 (4.8%) participants in the fluvoxamine group and 15 (5.5%) participants in the placebo group (absolute difference at day 15, 0.68%; 95% CI, −3.0% to 4.4%; log-rank P = .91).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>This trial did not find fluvoxamine efficacious in preventing clinical deterioration in unvaccinated outpatients with symptomatic COVID-19. It was stopped early and underpowered due to low primary outcome rates.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>ClinicalTrials.gov Identifier: NCT04668950.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3203-4590",
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Reiersen",
"given": "Angela M",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-8603-2916",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Mattar",
"given": "Caline",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6167-1447",
"affiliation": [
{
"name": "Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center , Seattle, Washington , USA"
},
{
"name": "Allergy & Infectious Diseases Division, Department of Medicine, University of Washington , Seattle, Washington , USA"
}
],
"authenticated-orcid": false,
"family": "Bender Ignacio",
"given": "Rachel A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, University of Minnesota , Minneapolis, Minnesota , USA"
}
],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2267-4239",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, McGill University Health Centre , Montréal, Québec , Canada"
},
{
"name": "Department of Medicine, Clinical Practice Assessment Unit, McGill University Health Centre , Montréal, Québec , Canada"
},
{
"name": "Division of Experimental Medicine, Department of Medicine, McGill University , Montréal, Québec , Canada"
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Todd C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Health System Innovation and Research, University of Utah , Salt Lake City, Utah , USA"
},
{
"name": "Division of General Internal Medicine, University of Utah , Salt Lake City, Utah , USA"
}
],
"family": "Hess",
"given": "Rachel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4769-7242",
"affiliation": [
{
"name": "Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center , Seattle, Washington , USA"
}
],
"authenticated-orcid": false,
"family": "Lankowski",
"given": "Alexander J",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0783-0624",
"affiliation": [
{
"name": "Department of Medicine, Clinical Practice Assessment Unit, McGill University Health Centre , Montréal, Québec , Canada"
},
{
"name": "Division of Experimental Medicine, Department of Medicine, McGill University , Montréal, Québec , Canada"
},
{
"name": "Division of General Internal Medicine, Department of Medicine, McGill University Health Centre , Montréal, Québec , Canada"
}
],
"authenticated-orcid": false,
"family": "McDonald",
"given": "Emily G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4568-6846",
"affiliation": [
{
"name": "Institute for Informatics, Data Science and Biostatistics, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Miller",
"given": "J Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Powderly",
"given": "William G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4644-7487",
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, University of Minnesota , Minneapolis, Minnesota , USA"
}
],
"authenticated-orcid": false,
"family": "Pullen",
"given": "Matthew F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departments of Psychiatry & Behavioral Sciences and Medicine, Northwestern University Feinberg School of Medicine , Chicago, Illinois , USA"
}
],
"family": "Rado",
"given": "Jeffrey T",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4243-9391",
"affiliation": [
{
"name": "Department of Medicine, Cardiovascular Division, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Rich",
"given": "Michael W",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2598-1621",
"affiliation": [
{
"name": "Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center , Seattle, Washington , USA"
},
{
"name": "Allergy & Infectious Diseases Division, Department of Medicine, University of Washington , Seattle, Washington , USA"
}
],
"authenticated-orcid": false,
"family": "Schiffer",
"given": "Joshua T",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7181-0305",
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Schweiger",
"given": "Julie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1815-7893",
"affiliation": [
{
"name": "Division of Infectious Diseases, University of Utah , Salt Lake City, Utah , USA"
}
],
"authenticated-orcid": false,
"family": "Spivak",
"given": "Adam M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Stevens",
"given": "Angela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2736-9639",
"affiliation": [
{
"name": "Department of Psychiatry, Temerty Faculty of Medicine, University of Toronto and Women's College Hospital , Toronto, Ontario , Canada"
}
],
"authenticated-orcid": false,
"family": "Vigod",
"given": "Simone N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family and Community Medicine, Temerty Faculty of Medicine, University of Toronto and Women's College Hospital , Toronto, Ontario , Canada"
}
],
"family": "Agarwal",
"given": "Payal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Yang",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Yingling",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Gettinger",
"given": "Torie R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"family": "Zorumski",
"given": "Charles F",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0471-9368",
"affiliation": [
{
"name": "Department of Psychiatry, Washington University School of Medicine , St. Louis, Missouri , USA"
},
{
"name": "Department of Anesthesiology, Washington University School of Medicine , St. Louis, Missouri , USA"
}
],
"authenticated-orcid": false,
"family": "Lenze",
"given": "Eric J",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T12:09:35Z",
"timestamp": 1691410175000
},
"deposited": {
"date-parts": [
[
2023,
8,
23
]
],
"date-time": "2023-08-23T18:29:57Z",
"timestamp": 1692815397000
},
"indexed": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T07:37:38Z",
"timestamp": 1711265858380
},
"is-referenced-by-count": 3,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
8,
1
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2023,
8,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 6,
"start": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T00:00:00Z",
"timestamp": 1691366400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofad419/51049817/ofad419.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/10/8/ofad419/51233785/ofad419.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/10/8/ofad419/51233785/ofad419.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
8,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
8
]
]
},
"published-other": {
"date-parts": [
[
2023,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
8,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review",
"author": "Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "JAMA",
"key": "2023082312394188400_ofad419-B1",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2023082312394188400_ofad419-B2",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"article-title": "Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis",
"author": "Rosen",
"doi-asserted-by": "crossref",
"first-page": "eaau5266",
"journal-title": "Sci Transl Med",
"key": "2023082312394188400_ofad419-B3",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1038/s41380-021-01309-5",
"article-title": "The acid sphingomyelinase/ceramide system in COVID-19",
"author": "Kornhuber",
"doi-asserted-by": "crossref",
"first-page": "307",
"journal-title": "Mol Psychiatry",
"key": "2023082312394188400_ofad419-B4",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "2023082312394188400_ofad419-B5",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.9223",
"article-title": "Clinical trials without clinical sites",
"author": "Cummings",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "JAMA Intern Med",
"key": "2023082312394188400_ofad419-B6",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"article-title": "Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e226269",
"journal-title": "JAMA Network Open",
"key": "2023082312394188400_ofad419-B7",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"journal-title": "Lancet Glob Health",
"key": "2023082312394188400_ofad419-B8",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "2023082312394188400_ofad419-B9",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.3389/fendo.2021.587801",
"article-title": "Metformin and Covid-19: focused review of mechanisms and current literature suggesting benefit",
"author": "Ibrahim",
"doi-asserted-by": "crossref",
"first-page": "587801",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "2023082312394188400_ofad419-B10",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1182/bloodadvances.2020003513",
"article-title": "Platelet reactivity to thrombin differs between patients with COVID-19 and those with ARDS unrelated to COVID-19",
"author": "Zaid",
"doi-asserted-by": "crossref",
"first-page": "635",
"journal-title": "Blood Adv",
"key": "2023082312394188400_ofad419-B11",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: a review of its mechanism of action and its role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"first-page": "652688",
"journal-title": "Front Pharmacol",
"key": "2023082312394188400_ofad419-B12",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.7326/M22-3305",
"article-title": "Oral fluvoxamine with inhaled budesonide for treatment of early-onset COVID-19: a randomized platform trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "Ann Intern Med",
"key": "2023082312394188400_ofad419-B13",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1001/jama.2022.24100",
"article-title": "Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "296",
"journal-title": "JAMA",
"key": "2023082312394188400_ofad419-B14",
"volume": "329",
"year": "2023"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofad419/7238414"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "The STOP COVID 2 Study: Fluvoxamine vs Placebo for Outpatients With Symptomatic COVID-19, a Fully Remote Randomized Controlled Trial",
"type": "journal-article",
"volume": "10"
}