The Real-World Effectiveness of Fluvoxamine Therapy in Mild to Moderate COVID-19 Patients; a Historical Cohort Study (Fluvoxa Trial)
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.10.010, Fluvoxa, TCTR20230401001, Oct 2023
31st treatment shown to reduce risk in
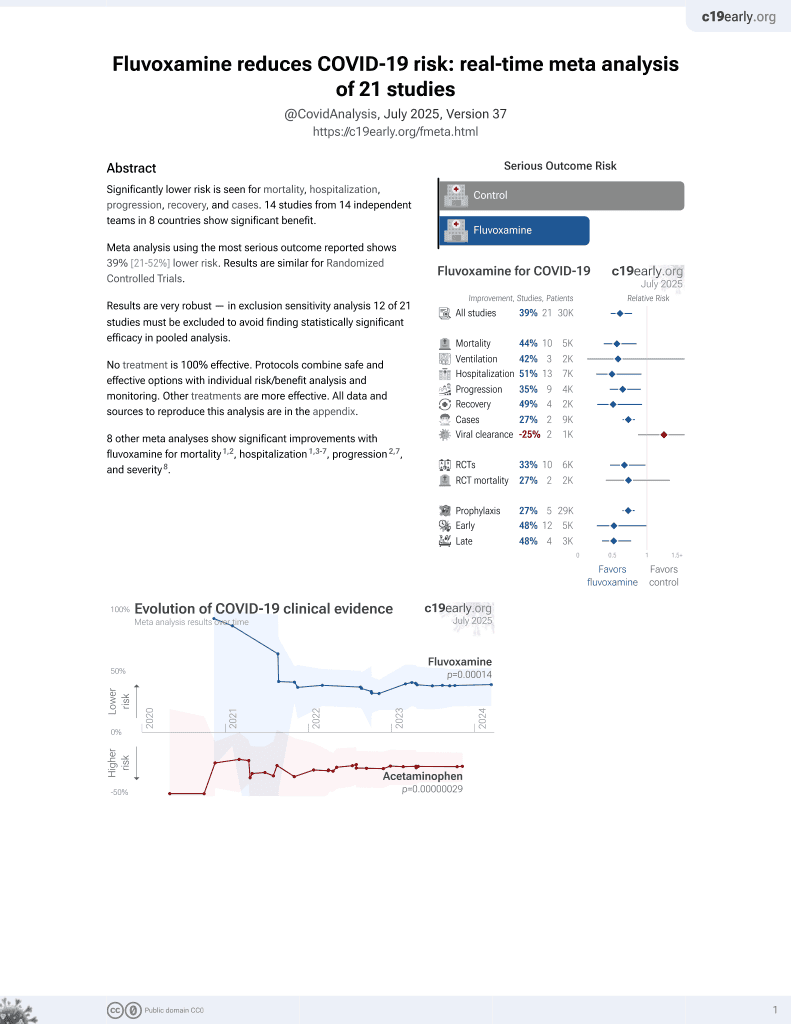
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 752 patients in Thailand showing mixed results with 50mg fluvoxamine bid. Authors note that trials showing benefit mostly used 100mg bid.
|
risk of death, 59.2% lower, RR 0.41, p = 1.00, treatment 0 of 234 (0.0%), control 1 of 518 (0.2%), NNT 518, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of mechanical ventilation, 47.6% higher, RR 1.48, p = 0.65, treatment 2 of 234 (0.9%), control 3 of 518 (0.6%).
|
|
risk of ICU admission, 26.2% lower, RR 0.74, p = 1.00, treatment 2 of 234 (0.9%), control 6 of 518 (1.2%), NNT 329.
|
|
risk of oxygen therapy, 67.3% higher, RR 1.67, p = 0.02, treatment 34 of 234 (14.5%), control 45 of 518 (8.7%).
|
|
risk of deterioration, 41.9% lower, RR 0.58, p = 0.08, treatment 13 of 217 (6.0%), control 49 of 475 (10.3%), NNT 23, day 14.
|
|
risk of deterioration, 35.0% lower, RR 0.65, p = 0.047, treatment 23 of 166 (13.9%), control 88 of 413 (21.3%), NNT 13, day 5.
|
|
risk of deterioration, 32.9% lower, RR 0.67, p = 0.004, treatment 51 of 217 (23.5%), control 132 of 377 (35.0%), NNT 8.7, day 2.
|
|
risk of progression, 47.6% higher, RR 1.48, p = 0.65, treatment 2 of 234 (0.9%), control 3 of 518 (0.6%), ARDS.
|
|
WHO-CPS, 33.6% lower, RR 0.66, p < 0.001, treatment mean 0.73 (±0.67) n=234, control mean 1.1 (±0.75) n=518, WHO-CPS score, day 14.
|
|
WHO-CPS, 7.9% higher, RR 1.08, p = 0.06, treatment mean 2.06 (±1.07) n=234, control mean 1.91 (±0.98) n=518, WHO-CPS score, day 5.
|
|
WHO-CPS, 3.3% higher, RR 1.03, p = 0.43, treatment mean 2.21 (±1.25) n=234, control mean 2.14 (±1.06) n=518, WHO-CPS score, day 2.
|
|
risk of no viral clearance, 3.6% higher, RR 1.04, p = 0.66, treatment 130 of 210 (61.9%), control 218 of 365 (59.7%), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Siripongboonsitti et al., 6 Oct 2023, retrospective, Thailand, peer-reviewed, 4 authors, study period 16 April, 2021 - 24 July, 2021, trial TCTR20230401001 (Fluvoxa).
Contact: taweegrit.sir@cra.ac.th.
The Real-World Effectiveness of Fluvoxamine Therapy in Mild to Moderate COVID-19 Patients; a Historical Cohort Study (Fluvoxa Trial)
Journal of Infection and Public Health, doi:10.1016/j.jiph.2023.10.010
Background: Fluvoxamine (FVX) has been proposed as a potential treatment for severe COVID-19 by the σ-1 receptor agonist, which can reduce cytokine production. However, the efficacy of FVX remains controversial.
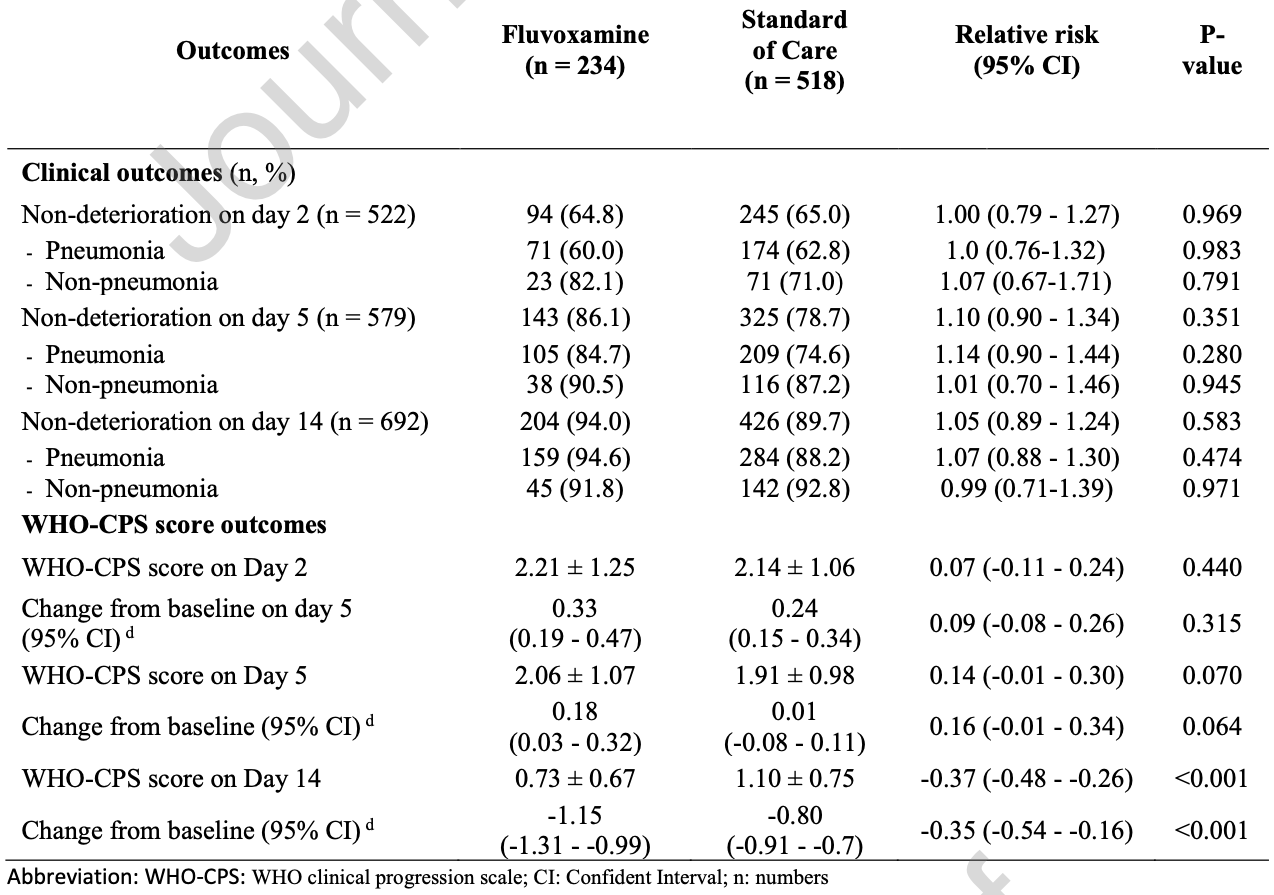
Methods: A historical retrospective cohort study was conducted in mild to moderate COVID-19 patients, 2:1 ratio of standard of care (SOC) and FVX treatments to assess the effectiveness of FVX in preventing deterioration by the fifth day of treatment. Results: Of 752 eligible patients, 234 received FVX while 518 received SOC, and there was no significant difference in the effectiveness of FVX and SOC in preventing clinical deterioration. On the fifth day after treatment, 86.1% of patients in the FVX-treated group did not experience clinical deterioration compared to 78.7% in the SOC group. Notably, the FVX group had higher rates of pneumonia development and hospitalization, requiring more oxygen supplementation while showing less reduction in viral shedding than the SOC group. However, no difference in mechanical ventilation use, ICU admission, and survival was observed.
Conclusion: Fluvoxamine treatment is failed to demonstrate effectiveness in preventing deterioration in mild to moderate COVID-19 and may lead to a higher incidence of pneumonia, hospitalization, and oxygen supplementation, necessitating careful consideration before prescribing the drug for COVID-19. Trial registration: Thai clinical trials registry (TCTR) no.
Consent for publication
Not applicable
Competing interests The authors declare that they have no competing interests.
Authors' Contributions T.S. had full access to all of the data in this study and took responsibility for the data's integrity and accuracy. T.S.-first authors. T.S.-Corresponding author, T.S., K.T., P.P., N.M. contributed equally to the study. Concept and design-T.S., N.M. Investigation-T.S., K.T., P.P., N.M., Acquisition, analysis, or interpretation of data: T.S.; Critical revision of the manuscript for important intellectual content: T.S.; Statistical analysis: A.K.; Obtained funding. N.M., T.S.; Administrative, technical, or material support: T.S.; Supervision: T.S., N.M.
Conflict of interest statement The authors have no interest to declare J o u r n a l P r e -p r o o f
References
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Calusic, Marcec, Luksa, Jurkovic, Kovac et al., Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls, Br J Clin Pharmacol, doi:10.1111/bcp.15126
Chuah, Chow, Hor, Cheng, Ker et al., Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized, Open-Label Clinical Trial, Clin Infect Dis, doi:10.1093/cid/ciab962
Fung, Liu, The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus, Virology, doi:10.1016/j.virol.2019.05.002
Guo, Harari, Chernecki, Thorlund, Forrest, Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials, Am J Trop Med Hyg, doi:10.4269/ajtmh.21-1310
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hassanipour, Arab-Zozani, Amani, Heidarzad, Fathalipour et al., The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials, Scientific reports, doi:10.1038/s41598-021-90551-6
Hoertel, Sánchez-Rico, De La Muela, Abellán, Blanco et al., Risk of Death in Individuals Hospitalized for COVID-19 With and Without Psychiatric Disorders: An Observational Multicenter Study in France, Biol Psychiatry Glob Open Sci, doi:10.1016/j.bpsgos.2021.12.007
Hoertel, Sánchez-Rico, Vernet, Beeker, Jannot et al., Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry, doi:10.1038/s41380-021-01021-4
Homolak, Kodvanj, Widely available lysosome targeting agents should be considered as potential therapy for COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106044
Horita, Fukumoto, Global case fatality rate from COVID-19 has decreased by 96.8% during 2.5 years of the pandemic, J Med Virol, doi:10.1002/jmv.28231
J O U R N A L P R E, None, doi:10.1186/s12985-020-01412-z
Lee, Vigod, Bortolussi-Courval, Hanula, Boulware et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.6269
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial, Jama, doi:10.1001/jama.2020.22760
Mccarthy, Naggie, Boulware, Lindsell, Stewart et al., Fluvoxamine for Outpatient Treatment of COVID-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial, medRxiv, doi:10.1101/2022.10.17.22281178.JournalPre-
Németh, Szûcs, Vitrai, Juhász, Németh et al., Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: A retrospective case-control study, Ideggyogy Sz, doi:10.18071/isz.74.0389
Oskotsky, Maric, Tang, Oskotsky, Wong et al., Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.33090.JournalPre-proof
Reis, Santos Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/s2214-109x(21)00448-4
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs, Clin Infect Dis, doi:10.1093/cid/ciac180.JournalPre-proof
Schlienger, Meier, Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?, Am J Cardiovasc Drugs, doi:10.2165/00129784-200303030-00001
Seftel, Boulware, Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19, Open Forum Infect Dis, doi:10.1093/ofid/ofab050
Seo, Kim, Bae, Park, Chung et al., Fluvoxamine Treatment of Patients with Symptomatic COVID-19 in a Community Treatment Center: A Preliminary Result of Randomized Controlled Trial, Infect Chemother, doi:10.3947/ic.2021.0142
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and metaanalysis, Virology journal, doi:10.1186/s12985-020-01412-z
Siripongboonsitti, Ungtrakul, Tawinprai, Nimmol, Buttakosa et al., Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study), Int J Infect Dis, doi:10.1016/j.ijid.2023.06.018
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.652688
Sánchez, Martínez-Sellés, García, Moreno Guillén, Rodríguez-Artalejo et al., Insights for COVID-19 in 2023, Rev Esp Quimioter, doi:10.37201/req/122.2022
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, Jama, doi:10.1001/jama.2020.2648
Zhou, Hill, Sarkar, Tse, Woodburn et al., β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, J Infect Dis, doi:10.1093/infdis/jiab247
Zhu, Zhang, Li, Yang, Song, A novel coronavirus from patients with pneumonia in China, New England Journal of Medicine, doi:10.1056/nejmoa2001017
DOI record:
{
"DOI": "10.1016/j.jiph.2023.10.010",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2023.10.010",
"alternative-id": [
"S1876034123003374"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "The Real-World Effectiveness of Fluvoxamine Therapy in Mild to Moderate COVID-19 Patients; a Historical Cohort Study (Fluvoxa Trial)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2023.10.010"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7256-9982",
"affiliation": [],
"authenticated-orcid": false,
"family": "Siripongboonsitti",
"given": "Taweegrit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tawinprai",
"given": "Kriangkrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Payoong",
"given": "Paruspak",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahanonda",
"given": "Nithi",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
7
]
],
"date-time": "2023-10-07T00:52:22Z",
"timestamp": 1696639942000
},
"deposited": {
"date-parts": [
[
2023,
10,
7
]
],
"date-time": "2023-10-07T08:55:42Z",
"timestamp": 1696668942000
},
"funder": [
{
"DOI": "10.13039/100016175",
"doi-asserted-by": "publisher",
"name": "Chulabhorn Royal Academy"
}
],
"indexed": {
"date-parts": [
[
2023,
10,
8
]
],
"date-time": "2023-10-08T19:15:19Z",
"timestamp": 1696792519868
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T00:00:00Z",
"timestamp": 1696118400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 3,
"start": {
"date-parts": [
[
2023,
10,
4
]
],
"date-time": "2023-10-04T00:00:00Z",
"timestamp": 1696377600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123003374?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1876034123003374?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
10
]
]
},
"published-print": {
"date-parts": [
[
2023,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.jiph.2023.10.010_bib1",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "Jama",
"key": "10.1016/j.jiph.2023.10.010_bib2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/jmv.28231",
"article-title": "Global case fatality rate from COVID-19 has decreased by 96.8% during 2.5 years of the pandemic",
"author": "Horita",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J Med Virol",
"key": "10.1016/j.jiph.2023.10.010_bib3",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2023.10.010_bib4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2023.10.010_bib5",
"volume": "386",
"year": "2022"
},
{
"key": "10.1016/j.jiph.2023.10.010_bib6",
"unstructured": "U.S. Food and Drug Administration, FDA NEWS RELEASE, Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19 2021 [updated December 22, 2021. Available from: 〈https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19〉."
},
{
"DOI": "10.1093/infdis/jiab247",
"article-title": "β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "415",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jiph.2023.10.010_bib7",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac180",
"article-title": "Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs",
"author": "Saravolatz",
"doi-asserted-by": "crossref",
"first-page": "165",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2023.10.010_bib8",
"volume": "76",
"year": "2023"
},
{
"key": "10.1016/j.jiph.2023.10.010_bib9",
"unstructured": "Department of Medical Service. Clinical Practice Guideline to Diagnosis, Treatment and Prevention COVID-19 for Physicain and Health Care Provider, 21 July 2021 2021 [Available from: 〈https://covid19.dms.go.th/Content/Select_Landding_page?contentId=139〉."
},
{
"DOI": "10.1038/s41598-021-90551-6",
"article-title": "The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials",
"author": "Hassanipour",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Scientific reports",
"key": "10.1016/j.jiph.2023.10.010_bib10",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Virology journal",
"key": "10.1016/j.jiph.2023.10.010_bib11",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab962",
"article-title": "Efficacy of Early Treatment With Favipiravir on Disease Progression Among High-Risk Patients With Coronavirus Disease 2019 (COVID-19): A Randomized, Open-Label Clinical Trial",
"author": "Chuah",
"doi-asserted-by": "crossref",
"first-page": "e432",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2023.10.010_bib12",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.652688",
"article-title": "Fluvoxamine: A Review of Its Mechanism of Action and Its Role in COVID-19",
"author": "Sukhatme",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.jiph.2023.10.010_bib13",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106044",
"article-title": "Widely available lysosome targeting agents should be considered as potential therapy for COVID-19",
"author": "Homolak",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.jiph.2023.10.010_bib14",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2019.05.002",
"article-title": "The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus",
"author": "Fung",
"doi-asserted-by": "crossref",
"first-page": "34",
"journal-title": "Virology",
"key": "10.1016/j.jiph.2023.10.010_bib15",
"volume": "533",
"year": "2019"
},
{
"DOI": "10.2165/00129784-200303030-00001",
"article-title": "Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction?",
"author": "Schlienger",
"doi-asserted-by": "crossref",
"first-page": "149",
"issue": "3",
"journal-title": "Am J Cardiovasc Drugs",
"key": "10.1016/j.jiph.2023.10.010_bib16",
"volume": "3",
"year": "2003"
},
{
"DOI": "10.37201/req/122.2022",
"article-title": "Insights for COVID-19 in 2023",
"author": "Martín Sánchez",
"doi-asserted-by": "crossref",
"first-page": "114",
"issue": "2",
"journal-title": "Rev Esp Quimioter",
"key": "10.1016/j.jiph.2023.10.010_bib17",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients With Symptomatic COVID-19: A Randomized Clinical Trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"issue": "22",
"journal-title": "Jama.",
"key": "10.1016/j.jiph.2023.10.010_bib18",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.21-1310",
"article-title": "Fluvoxamine for the Early Treatment of COVID-19: A Meta-analysis of Randomized Clinical Trials",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "1315",
"issue": "5",
"journal-title": "Am J Trop Med Hyg",
"key": "10.1016/j.jiph.2023.10.010_bib19",
"volume": "106",
"year": "2022"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"issue": "1",
"journal-title": "Lancet Glob Health",
"key": "10.1016/j.jiph.2023.10.010_bib20",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3947/ic.2021.0142",
"article-title": "Fluvoxamine Treatment of Patients with Symptomatic COVID-19 in a Community Treatment Center: A Preliminary Result of Randomized Controlled Trial",
"author": "Seo",
"doi-asserted-by": "crossref",
"first-page": "102",
"issue": "1",
"journal-title": "Infect Chemother",
"key": "10.1016/j.jiph.2023.10.010_bib21",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"article-title": "Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2023.10.010_bib22",
"volume": "5",
"year": "2022"
},
{
"article-title": "Fluvoxamine for Outpatient Treatment of COVID-19: A Decentralized, Placebo-controlled, Randomized, Platform Clinical Trial",
"author": "McCarthy",
"journal-title": "medRxiv",
"key": "10.1016/j.jiph.2023.10.010_bib23",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofab050",
"article-title": "Prospective Cohort of Fluvoxamine for Early Treatment of Coronavirus Disease 19",
"author": "Seftel",
"doi-asserted-by": "crossref",
"first-page": "ofab050",
"issue": "2",
"journal-title": "Open Forum Infect Dis",
"key": "10.1016/j.jiph.2023.10.010_bib24",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1111/bcp.15126",
"article-title": "Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls",
"author": "Calusic",
"doi-asserted-by": "crossref",
"first-page": "2065",
"issue": "5",
"journal-title": "Br J Clin Pharmacol",
"key": "10.1016/j.jiph.2023.10.010_bib25",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2023.06.018",
"article-title": "Efficacy of combination therapy of fluvoxamine and favipiravir vs favipiravir monotherapy to prevent severe COVID-19 among mild to moderate COVID-19 patients: Open-label randomized controlled trial (EFFaCo study)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"first-page": "211",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiph.2023.10.010_bib26",
"volume": "134",
"year": "2023"
},
{
"DOI": "10.18071/isz.74.0389",
"article-title": "Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: A retrospective case-control study",
"author": "Németh",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "11-12",
"journal-title": "Ideggyogy Sz",
"key": "10.1016/j.jiph.2023.10.010_bib27",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01021-4",
"article-title": "Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "5199",
"issue": "9",
"journal-title": "Mol Psychiatry",
"key": "10.1016/j.jiph.2023.10.010_bib28",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.bpsgos.2021.12.007",
"article-title": "Risk of Death in Individuals Hospitalized for COVID-19 With and Without Psychiatric Disorders: An Observational Multicenter Study in France",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "56",
"issue": "1",
"journal-title": "Biol Psychiatry Glob Open Sci",
"key": "10.1016/j.jiph.2023.10.010_bib29",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"article-title": "Mortality Risk Among Patients With COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants",
"author": "Oskotsky",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.jiph.2023.10.010_bib30",
"volume": "4",
"year": "2021"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1876034123003374"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "The Real-World Effectiveness of Fluvoxamine Therapy in Mild to Moderate COVID-19 Patients; a Historical Cohort Study (Fluvoxa Trial)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}