Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: a prospective interventional open-label cohort study
et al., Molecular Psychiatry, doi:10.1038/s41380-023-02004-3, Mar 2023
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
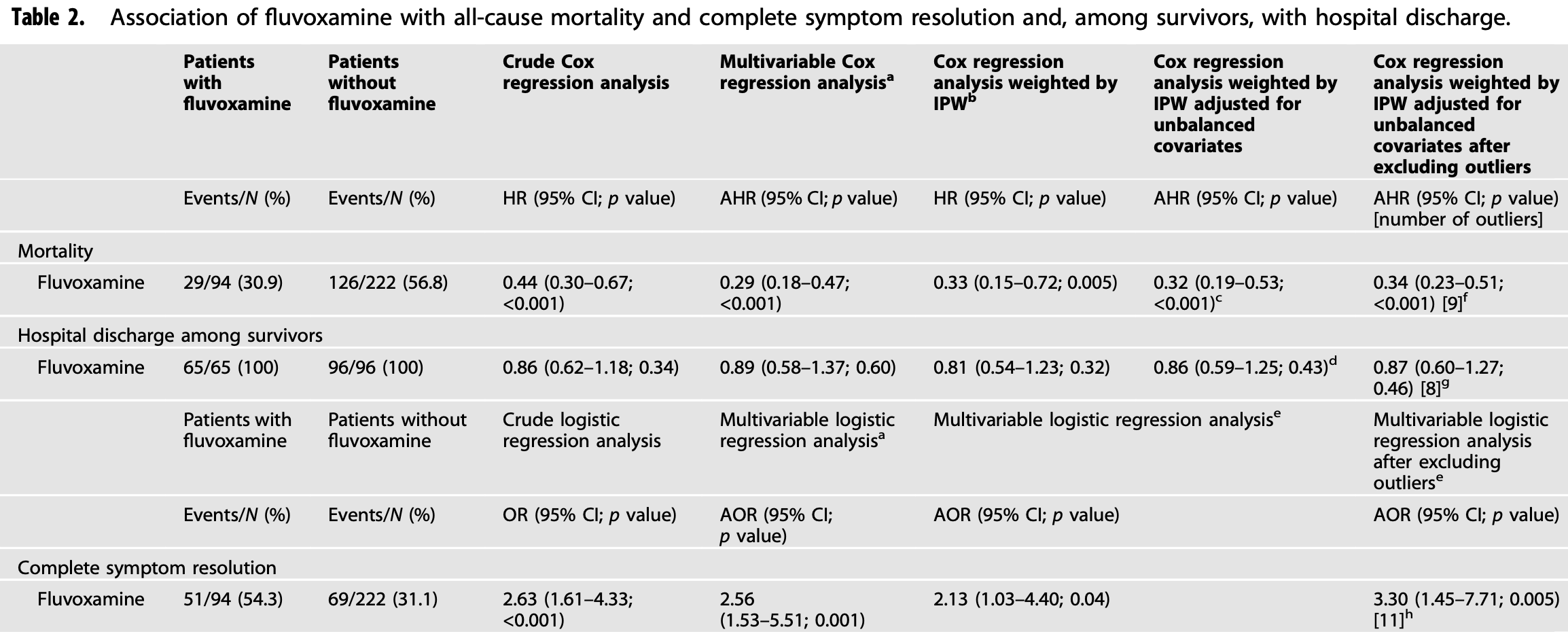
Prospective study of 316 hospitalized patients in Uganda, 94 receiving fluvoxamine, showing significantly lower mortality and improved recovery with treatment.
|
risk of death, 68.0% lower, HR 0.32, p < 0.001, treatment 29 of 94 (30.9%), control 126 of 222 (56.8%), NNT 3.9, adjusted for unbalanced covariates, propensity score weighting, Cox proportional hazards.
|
|
symptom resolution, 53.1% lower, HR 0.47, p = 0.04, treatment 94, control 222, inverted to make HR<1 favor treatment, propensity score weighting, Cox proportional hazards, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kirenga et al., 3 Mar 2023, prospective, Uganda, peer-reviewed, 19 authors, study period December 2021 - February 2022.
Contact: brucekirenga@yahoo.co.uk.
Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: a prospective interventional open-label cohort study
Molecular Psychiatry, doi:10.1038/s41380-023-02004-3
Prior research suggests that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) used for the treatment of obsessivecompulsive disorder and major depressive disorder, could be repurposed against COVID-19. We undertook a prospective interventional open-label cohort study to evaluate the efficacy and tolerability of fluvoxamine among inpatients with laboratory-confirmed COVID-19 in Uganda. The main outcome was all-cause mortality. Secondary outcomes were hospital discharge and complete symptom resolution. We included 316 patients, of whom 94 received fluvoxamine in addition to standard care [median age, 60 years (IQR = 37.0); women, 52.2%]. Fluvoxamine use was significantly associated with reduced mortality [AHR = 0.32; 95% CI = 0.19-0.53; p < 0.001, NNT = 4.46] and with increased complete symptom resolution [AOR = 2.56; 95% CI = 1.53-5.51; p < 0.001, NNT = 4.44]. Sensitivity analyses yielded similar results. These effects did not significantly differ by clinical characteristic, including vaccination status. Among the 161 survivors, fluvoxamine was not significantly associated with time to hospital discharge [AHR 0.81, 95% CI (0.54-1.23), p = 0.32]. There was a trend toward greater side effects with fluvoxamine (7.45% versus 3.15%; SMD = 0.21; χ 2 = 3.46, p = 0.06), most of which were light or mild in severity and none of which were serious. One hundred mg of fluvoxamine prescribed twice daily for 10 days was well tolerated and significantly associated with reduced mortality and with increased complete symptom resolution, without a significant increase in time to hospital discharge, among inpatients with COVID-19. Large-scale randomized trials are urgently needed to confirm these findings, especially for low-and middle-income countries, where access to vaccines and approved treatments against COVID-19 is limited.
AUTHOR CONTRIBUTIONS Concept and design: BJK; Funding acquisition: BJK and WB; Acquisition of data: RM, EM, EK, VN, LON, RS, WK and HA; Administrative, technical, or material support: WM, IS, HK, RB and HGM; Supervision: BJK and WM; Critical revision of the manuscript: BJK, LM, MSR, NH, WM, PBK and WB; Statistical analysis: BJK, LM, MSR and NH.
COMPETING INTERESTS NH is an inventor on a patent application related to methods of treating COVID-19 (FIASMA antidepressants), filled by Assistance Publique-Hopitaux de Paris in France. The other authors have no conflicts of interest related to this work to declare.
ETHICAL APPROVAL Compassionate use approval of fluvoxamine for the treatment of COVID-19 was obtained from the Ministry of Health of Uganda (clearance reference no. ADM.180/O1 dated 2nd January 2022). All patients were informed that this medication was under evaluation by health authorities. Patients willing to be treated with this medication gave verbal informed consent. All data used in this study were collected as part of routine care.
ADDITIONAL INFORMATION Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41380-023-02004-3. Correspondence and requests for materials should be addressed to Bruce J. Kirenga. Reprints and permission information is available at http://www.nature.com/ reprints Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and..
References
Austin, Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research, Commun Stat Simul Comput
Bramante, Huling, Tignanelli, Buse, Liebovitz et al., Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N Engl J Med
Brunotte, Zheng, Mecate-Zambrano, Tang, Ludwig et al., Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro, Pharmaceutics
Calusic, Marcec, Luksa, Jurkovic, Novac et al., Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls, Br J Clin Pharmacol, doi:10.1111/bcp.15126
Carpinteiro, Edwards, Hoffmann, Kochs, Gripp et al., Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells, Cell Rep Med
Carpinteiro, Gripp, Hoffmann, Pöhlmann, Hoertel et al., Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells, J Biol Chem
Chen, Wu, Chen, Zhan, Wu et al., Sertraline is an effective SARS-CoV-2 entry inhibitor targeting the spike protein, J Virol
Chevance, Gourion, Hoertel, Llorca, Thomas et al., Ensuring mental health care during the SARS-CoV-2 epidemic in France: a narrative review, L'Encéphale
Ciotti, Ciccozzi, Terrinoni, Jiang, Wang et al., The COVID-19 pandemic, Crit Rev Clin Lab Sci
Cipriani, Furukawa, Salanti, Chaimani, Ogawa et al., Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis, Lancet
Clelland, Ramiah, Steinberg, Clelland, Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort, BJPsych Open
Core, R: a language and environment for statistical computing
Court, The definition of acute respiratory illnesses in children, Postgrad Med J
Creeden, Imami, Eby, Gillman, Becker et al., Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection, Biomed Pharmacother
Dechaumes, Nekoua, Belouzard, Sane, Engelmann et al., Fluoxetine can inhibit SARS-CoV-2 in vitro, Microorganisms
Deng, Rayner, Ramaraju, Abbas, García et al., Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis, Clin Microbiol Infect
Efron, Nonparametric standard errors and confidence intervals, Can J Stat
Fred, Kuivanen, Ugurlu, Casarotto, Levanov et al., Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro, Front Pharm
Fritz, Hoertel, Lenze, Jalali, Reiersen, Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2, Transl Psychiatry
Grambsch, Therneau, Proportional hazards tests and diagnostics based on weighted residuals, Biometrika
Gulbins, Palmada, Reichel, Lüth, Böhmer et al., Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs, Nat Med
Hashimoto, Suzuki, Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: a historical review, Mol Psychiatry
Ho, Imai, King, Stuart, MatchIt: nonparametric preprocessing for parametric causal inference, J Stat Softw, doi:10.18637/jss.v042.i08
Hoertel, Blachier, Blanco, Olfson, Masseti et al., A stochastic agent-based model of the SARS-CoV-2 epidemic in France, Nat Med
Hoertel, Boulware, Sánchez-Rico, Burgun, Limosin, Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease, JAMA Netw Open
Hoertel, Do the selective serotonin reuptake inhibitor antidepressants fluoxetine and fluvoxamine reduce mortality among patients with COVID-19, JAMA Netw Open
Hoertel, Sánchez-Rico, Cougoule, Gulbins, Kornhuber et al., Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms, Mol Psychiatry
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Carpinteiro et al., Association between FIASMA psychotropic medications and reduced risk of intubation or death in individuals with psychiatric disorders hospitalized for severe COVID-19: an observational multicenter study, Transl Psychiatry
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Carpinteiro et al., Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study, Clin Pharm Ther
Hoertel, Sánchez-Rico, Gulbins, Kornhuber, Vernet et al., Association between benzodiazepine receptor agonist use and mortality in patients hospitalised for COVID-19: a multicentre observational study, Epidemiol Psychiatr Sci
Hoertel, Sánchez-Rico, Herrera-Morueco, De La Muela, Gulbins et al., Comorbid medical conditions are a key factor to understand the relationship between psychiatric disorders and COVID-19-related mortality: results from 49,089 COVID-19 inpatients, Mol Psychiatry
Hoertel, Sánchez-Rico, Kornhuber, Gulbins, Reiersen et al., Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19, JCM
Hoertel, Sánchez-Rico, Vernet, Beeker, Jannot et al., Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry
Hoertel, Sánchez-Rico, Vernet, Jannot, Neuraz et al., Observational study of chlorpromazine in hospitalized patients with COVID-19, Clin Drug Investig
Hoertel, Sánchez-Rico, Vernet, Jannot, Neuraz et al., Observational study of haloperidol in hospitalized patients with COVID-19, PLoS ONE
Ishima, Fujita, Hashimoto, Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells, Eur J Pharmacol
Kassambara, Kosinski, Biecek, Survminer: Drawing Survival Curves Using "Ggplot2
Keehner, Horton, Pfeffer, Longhurst, Schooley et al., SARS-CoV-2 infection after vaccination in health care workers in California, N Engl J Med
Khater, El-Khouly, Hm, Am, Ghorab, Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection, Int J Pharm
Kirenga, Muttamba, Kayongo, Nsereko, Siddharthan et al., Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda, BMJ Open Resp Res
Kornhuber, Hoertel, Gulbins, The acid sphingomyelinase/ceramide system in COVID-19, Mol Psychiatry
Lee, Vigod, Bortolussi-Courval, Hanula, Boulware et al., Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis, JAMA Netw Open
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Lim, Tignanelli, Hoertel, Boulware, Usher, Prevalence of medical contraindications to nirmatrelvir/ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19, Open Forum Infect Dis
Marcec, Dodig, Likic, A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference, J Infect
Musoke, Okot, Nanfuka, Rwamafa, Masajjage et al., A preliminary report on herbal medicine use among patients hospitalized at two-large COVID-19 treatment centers in Uganda, RMHP
Oskotsky, Maric, Tang, Oskotsky, Wong et al., Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants, JAMA Netw Open
Palmer, Benfield, Fluvoxamine: an overview of its pharmacological properties and review of its therapeutic potential in non-depressive disorders, CNS Drugs
Pharmaceuticals, Luvox (fluvoxamine maleate)-full prescribing information
Péricat, Sa, Sánchez-Rico, Mühle, Zoicas et al., Antiviral and anti-inflammatory activities of fluoxetine in a SARS-CoV-2 infection mouse model, Int J Mol Sci
Ranganathan, Pramesh, Aggarwal, Common pitfalls in statistical analysis: absolute risk reduction, relative risk reduction, and number needed to treat, Perspect Clin Res
Reis, Mills, Fluvoxamine for the treatment of COVID-19-author's reply, Lancet Glob Health
Reis, Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/S2214-109X(21)00448-4
Robins, Hernán, Brumback, Marginal structural models and causal inference in epidemiology, Epidemiology
Rosen, Seki, Fernández-Castañeda, Beiter, Eccles et al., Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis, Sci Transl Med
Schloer, Brunotte, Goretzko, Mecate-Zambrano, Korthals et al., Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine, Emerg Microbes Infect
Schloer, Brunotte, Mecate-Zambrano, Zheng, Tang et al., Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro, Br J Pharm
Schmidt, Kruse, The molecular function of σ receptors: past, present, and future, Trends Pharmacol Sci
Seftel, Boulware, Prospective cohort of fluvoxamine for early treatment of Coronavirus Disease 19, Open Forum Infect Dis
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxamine: a review of its mechanism of action and its role in COVID-19, Front Pharm
Therneau, Grambsch, Modeling survival data: extending the Cox model
Uyeki, Influenza, None, Ann Intern Med
Wang, Paulson, Pease, Watson, Comfort et al., Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21, Lancet
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann Med
Yang, Nnt: the number needed to treat (NNT) for survival endpoint
Zimniak, Kirschner, Hilpert, Geiger, Danov et al., The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue, Sci Rep
DOI record:
{
"DOI": "10.1038/s41380-023-02004-3",
"ISSN": [
"1359-4184",
"1476-5578"
],
"URL": "http://dx.doi.org/10.1038/s41380-023-02004-3",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Prior research suggests that fluvoxamine, a selective serotonin reuptake inhibitor (SSRI) used for the treatment of obsessive-compulsive disorder and major depressive disorder, could be repurposed against COVID-19. We undertook a prospective interventional open-label cohort study to evaluate the efficacy and tolerability of fluvoxamine among inpatients with laboratory-confirmed COVID-19 in Uganda. The main outcome was all-cause mortality. Secondary outcomes were hospital discharge and complete symptom resolution. We included 316 patients, of whom 94 received fluvoxamine in addition to standard care [median age, 60 years (IQR = 37.0); women, 52.2%]. Fluvoxamine use was significantly associated with reduced mortality [AHR = 0.32; 95% CI = 0.19–0.53; <jats:italic>p</jats:italic> < 0.001, NNT = 4.46] and with increased complete symptom resolution [AOR = 2.56; 95% CI = 1.53–5.51; <jats:italic>p</jats:italic> < 0.001, NNT = 4.44]. Sensitivity analyses yielded similar results. These effects did not significantly differ by clinical characteristic, including vaccination status. Among the 161 survivors, fluvoxamine was not significantly associated with time to hospital discharge [AHR 0.81, 95% CI (0.54–1.23), <jats:italic>p</jats:italic> = 0.32]. There was a trend toward greater side effects with fluvoxamine (7.45% versus 3.15%; SMD = 0.21; <jats:italic>χ</jats:italic><jats:sup>2</jats:sup> = 3.46, <jats:italic>p</jats:italic> = 0.06), most of which were light or mild in severity and none of which were serious. One hundred mg of fluvoxamine prescribed twice daily for 10 days was well tolerated and significantly associated with reduced mortality and with increased complete symptom resolution, without a significant increase in time to hospital discharge, among inpatients with COVID-19. Large-scale randomized trials are urgently needed to confirm these findings, especially for low- and middle-income countries, where access to vaccines and approved treatments against COVID-19 is limited.</jats:p>",
"alternative-id": [
"2004"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "17 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "8 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "15 February 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "3 March 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "NH is an inventor on a patent application related to methods of treating COVID-19 (FIASMA antidepressants), filled by Assistance Publique-Hopitaux de Paris in France. The other authors have no conflicts of interest related to this work to declare."
},
{
"group": {
"label": "Ethical approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Compassionate use approval of fluvoxamine for the treatment of COVID-19 was obtained from the Ministry of Health of Uganda (clearance reference no. ADM.180/O1 dated 2nd January 2022). All patients were informed that this medication was under evaluation by health authorities. Patients willing to be treated with this medication gave verbal informed consent. All data used in this study were collected as part of routine care."
}
],
"author": [
{
"affiliation": [],
"family": "Kirenga",
"given": "Bruce J.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9423-2472",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mugenyi",
"given": "Levicatus",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1121-8641",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sánchez-Rico",
"given": "Marina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6593-8727",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kyobe",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muttamba",
"given": "Winters",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mugume",
"given": "Raymond",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mwesigwa",
"given": "Eliya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalimo",
"given": "Ezra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nyombi",
"given": "Vicky",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4535-1694",
"affiliation": [],
"authenticated-orcid": false,
"family": "Segawa",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Namakula",
"given": "Loryndah Olive",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sekibira",
"given": "Rogers",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kabweru",
"given": "Wilberforce",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Byanyima",
"given": "Rosemary",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3159-4318",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aanyu",
"given": "Hellen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0757-1968",
"affiliation": [],
"authenticated-orcid": false,
"family": "Byakika-Kibwika",
"given": "Pauline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mwebesa",
"given": "Henry G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoertel",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bazeyo",
"given": "William",
"sequence": "additional"
}
],
"container-title": "Molecular Psychiatry",
"container-title-short": "Mol Psychiatry",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
3
]
],
"date-time": "2023-03-03T09:02:28Z",
"timestamp": 1677834148000
},
"deposited": {
"date-parts": [
[
2023,
3,
3
]
],
"date-time": "2023-03-03T10:05:59Z",
"timestamp": 1677837959000
},
"funder": [
{
"name": "Government of Uganda through Makerere University Research and Innovations Fund"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
4
]
],
"date-time": "2023-03-04T05:44:07Z",
"timestamp": 1677908647897
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
3
]
],
"date-time": "2023-03-03T00:00:00Z",
"timestamp": 1677801600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
3
]
],
"date-time": "2023-03-03T00:00:00Z",
"timestamp": 1677801600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41380-023-02004-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41380-023-02004-3",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41380-023-02004-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
3,
3
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
3
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1080/10408363.2020.1783198",
"author": "M Ciotti",
"doi-asserted-by": "publisher",
"first-page": "365",
"journal-title": "Crit Rev Clin Lab Sci",
"key": "2004_CR1",
"unstructured": "Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57:365–88.",
"volume": "57",
"year": "2020"
},
{
"DOI": "10.1016/j.encep.2020.04.005",
"author": "A Chevance",
"doi-asserted-by": "publisher",
"first-page": "193",
"journal-title": "L’Encéphale",
"key": "2004_CR2",
"unstructured": "Chevance A, Gourion D, Hoertel N, Llorca PM, Thomas P, Bocher P, et al. Ensuring mental health care during the SARS-CoV-2 epidemic in France: a narrative review. L’Encéphale. 2020;46:193–201.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1001-6",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "1417",
"journal-title": "Nat Med",
"key": "2004_CR3",
"unstructured": "Hoertel N, Blachier M, Blanco C, Olfson M, Masseti M, Sánchez-Rico M, et al. A stochastic agent-based model of the SARS-CoV-2 epidemic in France. Nat Med. 2020;26:1417–21.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)02796-3",
"author": "H Wang",
"doi-asserted-by": "publisher",
"first-page": "1513",
"journal-title": "Lancet",
"key": "2004_CR4",
"unstructured": "Wang H, Paulson KR, Pease SA, Watson S, Comfort H, Zheng P, et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399:1513–36.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.2147/RMHP.S339408",
"author": "P Musoke",
"doi-asserted-by": "publisher",
"first-page": "4609",
"journal-title": "RMHP",
"key": "2004_CR5",
"unstructured": "Musoke P, Okot J, Nanfuka V, Rwamafa P, Masajjage J, Kisuule I, et al. A preliminary report on herbal medicine use among patients hospitalized at two-large COVID-19 treatment centers in Uganda. RMHP. 2021;14:4609–17.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2101927",
"author": "J Keehner",
"doi-asserted-by": "publisher",
"first-page": "1774",
"journal-title": "N Engl J Med",
"key": "2004_CR6",
"unstructured": "Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley R, Currier JS, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–5.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofac389",
"author": "S Lim",
"doi-asserted-by": "publisher",
"first-page": "ofac389",
"journal-title": "Open Forum Infect Dis",
"key": "2004_CR7",
"unstructured": "Lim S, Tignanelli CJ, Hoertel N, Boulware DR, Usher MG. Prevalence of medical contraindications to nirmatrelvir/ritonavir in a cohort of hospitalized and nonhospitalized patients with COVID-19. Open Forum Infect Dis. 2022;9:ofac389.",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2022.42140",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "e2242140",
"journal-title": "JAMA Netw Open",
"key": "2004_CR8",
"unstructured": "Hoertel N, Boulware DR, Sánchez-Rico M, Burgun A, Limosin F. Prevalence of contraindications to nirmatrelvir-ritonavir among hospitalized patients with COVID-19 at risk for progression to severe disease. JAMA Netw Open. 2022;5:e2242140.",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.36510",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "e2136510",
"journal-title": "JAMA Netw Open",
"key": "2004_CR9",
"unstructured": "Hoertel N. Do the selective serotonin reuptake inhibitor antidepressants fluoxetine and fluvoxamine reduce mortality among patients with COVID-19. JAMA Netw Open. 2021;4:e2136510.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01393-7",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "1278",
"journal-title": "Mol Psychiatry",
"key": "2004_CR10",
"unstructured": "Hoertel N, Sánchez-Rico M, Herrera-Morueco JJ, de la Muela P, Gulbins E, Kornhuber J, et al. Comorbid medical conditions are a key factor to understand the relationship between psychiatric disorders and COVID-19-related mortality: results from 49,089 COVID-19 inpatients. Mol Psychiatry. 2022;27:1278–80.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41380-021-01254-3",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "7098",
"journal-title": "Mol Psychiatry",
"key": "2004_CR11",
"unstructured": "Hoertel N, Sánchez-Rico M, Cougoule C, Gulbins E, Kornhuber J, Carpinteiro A, et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry. 2021;26:7098–9.",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.2165/00023210-199401010-00006",
"author": "KJ Palmer",
"doi-asserted-by": "publisher",
"first-page": "57",
"journal-title": "CNS Drugs",
"key": "2004_CR12",
"unstructured": "Palmer KJ, Benfield P. Fluvoxamine: an overview of its pharmacological properties and review of its therapeutic potential in non-depressive disorders. CNS Drugs. 1994;1:57–87.",
"volume": "1",
"year": "1994"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"author": "TC Lee",
"doi-asserted-by": "publisher",
"first-page": "e226269",
"journal-title": "JAMA Netw Open",
"key": "2004_CR13",
"unstructured": "Lee TC, Vigod S, Bortolussi-Courval É, Hanula R, Boulware DR, Lenze EJ, et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e226269.",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"author": "W Wen",
"doi-asserted-by": "publisher",
"first-page": "516",
"journal-title": "Ann Med",
"key": "2004_CR14",
"unstructured": "Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54:516–23.",
"volume": "54",
"year": "2022"
},
{
"author": "R Marcec",
"first-page": "00672",
"journal-title": "J Infect",
"key": "2004_CR15",
"unstructured": "Marcec R, Dodig VM, Likic R. A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference. J Infect. 2022;S0163-4453:00672–7.",
"volume": "S0163-4453",
"year": "2022"
},
{
"author": "J Deng",
"first-page": "00032",
"journal-title": "Clin Microbiol Infect",
"key": "2004_CR16",
"unstructured": "Deng J, Rayner D, Ramaraju HB, Abbas U, García C, Heybati K, et al. Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: a systematic review and meta-analysis. Clin Microbiol Infect. 2023;S1198-743X:00032–0.",
"volume": "S1198-743X",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofab050",
"author": "D Seftel",
"doi-asserted-by": "publisher",
"first-page": "ofab050",
"journal-title": "Open Forum Infect Dis",
"key": "2004_CR17",
"unstructured": "Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of Coronavirus Disease 19. Open Forum Infect Dis. 2021;8:ofab050.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"doi-asserted-by": "publisher",
"key": "2004_CR18",
"unstructured": "Reis G, dos Santos Moreira-Silva EA, Silva DCM, Thabane L, Milagres AC, dos Santos CVQ, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2021:S2214109X21004484. https://doi.org/10.1016/S2214-109X(21)00448-4."
},
{
"DOI": "10.1001/jama.2020.22760",
"author": "EJ Lenze",
"doi-asserted-by": "publisher",
"first-page": "2292",
"journal-title": "JAMA",
"key": "2004_CR19",
"unstructured": "Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–300.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1111/bcp.15126",
"doi-asserted-by": "publisher",
"key": "2004_CR20",
"unstructured": "Calusic M, Marcec R, Luksa L, Jurkovic I, Novac N, Mihaljevic S, et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15126."
},
{
"DOI": "10.1056/NEJMoa2201662",
"author": "CT Bramante",
"doi-asserted-by": "publisher",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "2004_CR21",
"unstructured": "Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19. N Engl J Med. 2022;387:599–610.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2020.1829082",
"author": "S Schloer",
"doi-asserted-by": "publisher",
"first-page": "2245",
"journal-title": "Emerg Microbes Infect",
"key": "2004_CR22",
"unstructured": "Schloer S, Brunotte L, Goretzko J, Mecate-Zambrano A, Korthals N, Gerke V, et al. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9:2245–55.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.xcrm.2020.100142",
"author": "A Carpinteiro",
"doi-asserted-by": "publisher",
"first-page": "100142",
"journal-title": "Cell Rep Med",
"key": "2004_CR23",
"unstructured": "Carpinteiro A, Edwards MJ, Hoffmann M, Kochs G, Gripp B, Weigang S, et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep Med. 2020;1:100142.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"author": "DA Rosen",
"doi-asserted-by": "publisher",
"first-page": "eaau5266",
"journal-title": "Sci Transl Med",
"key": "2004_CR24",
"unstructured": "Rosen DA, Seki SM, Fernández-Castañeda A, Beiter R, Eccles J, Woodfolk JA, et al. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11:eaau5266.",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.3389/fphar.2021.652688",
"author": "VP Sukhatme",
"doi-asserted-by": "publisher",
"first-page": "652688",
"journal-title": "Front Pharm",
"key": "2004_CR25",
"unstructured": "Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharm. 2021;12:652688.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01432-3",
"author": "Y Hashimoto",
"doi-asserted-by": "publisher",
"first-page": "1898",
"journal-title": "Mol Psychiatry",
"key": "2004_CR26",
"unstructured": "Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022;27:1898–907.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41380-021-01309-5",
"author": "J Kornhuber",
"doi-asserted-by": "publisher",
"first-page": "307",
"journal-title": "Mol Psychiatry",
"key": "2004_CR27",
"unstructured": "Kornhuber J, Hoertel N, Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2022;27:307–14.",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1136/pgmj.49.577.771",
"author": "SDM Court",
"doi-asserted-by": "publisher",
"first-page": "771",
"journal-title": "Postgrad Med J",
"key": "2004_CR28",
"unstructured": "Court SDM. The definition of acute respiratory illnesses in children. Postgrad Med J. 1973;49:771–6.",
"volume": "49",
"year": "1973"
},
{
"DOI": "10.1016/j.tips.2019.07.006",
"author": "HR Schmidt",
"doi-asserted-by": "publisher",
"first-page": "636",
"journal-title": "Trends Pharmacol Sci",
"key": "2004_CR29",
"unstructured": "Schmidt HR, Kruse AC. The molecular function of σ receptors: past, present, and future. Trends Pharmacol Sci. 2019;40:636–54.",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.1016/j.ejphar.2014.01.064",
"author": "T Ishima",
"doi-asserted-by": "publisher",
"first-page": "167",
"journal-title": "Eur J Pharmacol",
"key": "2004_CR30",
"unstructured": "Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–73.",
"volume": "727",
"year": "2014"
},
{
"DOI": "10.1038/nm.3214",
"author": "E Gulbins",
"doi-asserted-by": "publisher",
"first-page": "934",
"journal-title": "Nat Med",
"key": "2004_CR31",
"unstructured": "Gulbins E, Palmada M, Reichel M, Lüth A, Böhmer C, Amato D, et al. Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. Nat Med. 2013;19:934–8.",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1016/j.jbc.2021.100701",
"author": "A Carpinteiro",
"doi-asserted-by": "publisher",
"first-page": "100701",
"journal-title": "J Biol Chem",
"key": "2004_CR32",
"unstructured": "Carpinteiro A, Gripp B, Hoffmann M, Pöhlmann S, Hoertel N, Edwards MJ, et al. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J Biol Chem. 2021;296:100701.",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2317",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "1498",
"journal-title": "Clin Pharm Ther",
"key": "2004_CR33",
"unstructured": "Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Lenze EJ, et al. Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharm Ther. 2021;110:1498–511.",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01021-4",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "5199",
"journal-title": "Mol Psychiatry",
"key": "2004_CR34",
"unstructured": "Hoertel N, Sánchez-Rico M, Vernet R, Beeker N, Jannot AS, Neuraz A, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26:5199–212.",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/s41398-022-01804-5",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"journal-title": "Transl Psychiatry",
"key": "2004_CR35",
"unstructured": "Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Carpinteiro A, Abellán M, et al. Association between FIASMA psychotropic medications and reduced risk of intubation or death in individuals with psychiatric disorders hospitalized for severe COVID-19: an observational multicenter study. Transl Psychiatry. 2022;12:90.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/jcm11195882",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "5882",
"journal-title": "JCM",
"key": "2004_CR36",
"unstructured": "Hoertel N, Sánchez-Rico M, Kornhuber J, Gulbins E, Reiersen AM, Lenze E, et al. Antidepressant use and its association with 28-day mortality in inpatients with SARS-CoV-2: support for the FIASMA model against COVID-19. JCM. 2022;11:5882.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"author": "T Oskotsky",
"doi-asserted-by": "publisher",
"first-page": "e2133090",
"journal-title": "JAMA Netw Open",
"key": "2004_CR37",
"unstructured": "Oskotsky T, Maric I, Tang A, Oskotsky B, Wong RJ, Aghaeepour N, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4:e2133090.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1038/s41398-022-02109-3",
"author": "BA Fritz",
"doi-asserted-by": "publisher",
"journal-title": "Transl Psychiatry",
"key": "2004_CR38",
"unstructured": "Fritz BA, Hoertel N, Lenze EJ, Jalali F, Reiersen AM. Association between antidepressant use and ED or hospital visits in outpatients with SARS-CoV-2. Transl Psychiatry. 2022;12:341.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.755600",
"author": "SM Fred",
"doi-asserted-by": "publisher",
"first-page": "755600",
"journal-title": "Front Pharm",
"key": "2004_CR39",
"unstructured": "Fred SM, Kuivanen S, Ugurlu H, Casarotto PC, Levanov L, Saksela K, et al. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front Pharm. 2022;12:755600.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1128/jvi.01245-22",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"first-page": "e0124522",
"journal-title": "J Virol",
"key": "2004_CR40",
"unstructured": "Chen Y, Wu Y, Chen S, Zhan Q, Wu D, Yang C, et al. Sertraline is an effective SARS-CoV-2 entry inhibitor targeting the spike protein. J Virol. 2022;96:e0124522.",
"volume": "96",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2021.121023",
"author": "SE Khater",
"doi-asserted-by": "publisher",
"first-page": "121023",
"journal-title": "Int J Pharm",
"key": "2004_CR41",
"unstructured": "Khater SE, El-khouly A, Abdel-Bar HM, Al-mahallawi AM, Ghorab DM. Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection. Int J Pharm. 2021;607:121023.",
"volume": "607",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics13091400",
"author": "L Brunotte",
"doi-asserted-by": "publisher",
"first-page": "1400",
"journal-title": "Pharmaceutics",
"key": "2004_CR42",
"unstructured": "Brunotte L, Zheng S, Mecate-Zambrano A, Tang J, Ludwig S, Rescher U, et al. Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro. Pharmaceutics. 2021;13:1400.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/microorganisms9020339",
"author": "A Dechaumes",
"doi-asserted-by": "publisher",
"first-page": "339",
"journal-title": "Microorganisms",
"key": "2004_CR43",
"unstructured": "Dechaumes A, Nekoua MP, Belouzard S, Sane F, Engelmann I, Dubuisson J, et al. Fluoxetine can inhibit SARS-CoV-2 in vitro. Microorganisms. 2021;9:339.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1111/bph.15418",
"author": "S Schloer",
"doi-asserted-by": "publisher",
"first-page": "2339",
"journal-title": "Br J Pharm",
"key": "2004_CR44",
"unstructured": "Schloer S, Brunotte L, Mecate-Zambrano A, Zheng S, Tang J, Ludwig S, et al. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br J Pharm. 2021;178:2339–50.",
"volume": "178",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-85049-0",
"author": "M Zimniak",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "2004_CR45",
"unstructured": "Zimniak M, Kirschner L, Hilpert H, Geiger N, Danov O, Oberwinkler H, et al. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci Rep. 2021;11:5890.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/ijms232113623",
"author": "D Péricat",
"doi-asserted-by": "publisher",
"first-page": "13623",
"journal-title": "Int J Mol Sci",
"key": "2004_CR46",
"unstructured": "Péricat D, Leon-Icaza SA, Sánchez-Rico M, Mühle C, Zoicas I, Schumacher F, et al. Antiviral and anti-inflammatory activities of fluoxetine in a SARS-CoV-2 infection mouse model. Int J Mol Sci. 2022;23:13623.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(17)32802-7",
"author": "A Cipriani",
"doi-asserted-by": "publisher",
"first-page": "1357",
"journal-title": "Lancet.",
"key": "2004_CR47",
"unstructured": "Cipriani A, Furukawa TA, Salanti G, Chaimani A, Ogawa Y, Leucht S, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66.",
"volume": "391",
"year": "2018"
},
{
"DOI": "10.1136/bmjresp-2020-000646",
"author": "B Kirenga",
"doi-asserted-by": "publisher",
"first-page": "e000646",
"journal-title": "BMJ Open Resp Res",
"key": "2004_CR48",
"unstructured": "Kirenga B, Muttamba W, Kayongo A, Nsereko C, Siddharthan T, Luisba J, et al. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Resp Res. 2020;7:e000646.",
"volume": "7",
"year": "2020"
},
{
"key": "2004_CR49",
"unstructured": "Jazz Pharmaceuticals. Luvox (fluvoxamine maleate)—full prescribing information. 2008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/022235lbl.pdf."
},
{
"DOI": "10.7326/AITC202111160",
"author": "TM Uyeki",
"doi-asserted-by": "publisher",
"first-page": "ITC161",
"journal-title": "Ann Intern Med",
"key": "2004_CR50",
"unstructured": "Uyeki TM. Influenza. Ann Intern Med. 2021;174:ITC161–76.",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1080/03610910902859574",
"author": "PC Austin",
"doi-asserted-by": "publisher",
"first-page": "1228",
"journal-title": "Commun Stat Simul Comput",
"key": "2004_CR51",
"unstructured": "Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–34.",
"volume": "38",
"year": "2009"
},
{
"DOI": "10.1097/00001648-200009000-00011",
"author": "JM Robins",
"doi-asserted-by": "publisher",
"first-page": "550",
"journal-title": "Epidemiology",
"key": "2004_CR52",
"unstructured": "Robins JM, Hernán MÁ, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–60.",
"volume": "11",
"year": "2000"
},
{
"DOI": "10.1017/S2045796021000743",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "e18",
"journal-title": "Epidemiol Psychiatr Sci",
"key": "2004_CR53",
"unstructured": "Hoertel N, Sánchez-Rico M, Gulbins E, Kornhuber J, Vernet R, Beeker N, et al. Association between benzodiazepine receptor agonist use and mortality in patients hospitalised for COVID-19: a multicentre observational study. Epidemiol Psychiatr Sci. 2022;31:e18.",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.1007/s40261-021-01001-0",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "221",
"journal-title": "Clin Drug Investig",
"key": "2004_CR54",
"unstructured": "Hoertel N, Sánchez-Rico M, Vernet R, Jannot AS, Neuraz A, Blanco C, et al. Observational study of chlorpromazine in hospitalized patients with COVID-19. Clin Drug Investig. 2021;41:221–33.",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0247122",
"author": "N Hoertel",
"doi-asserted-by": "publisher",
"first-page": "e0247122",
"journal-title": "PLoS ONE",
"key": "2004_CR55",
"unstructured": "Hoertel N, Sánchez-Rico M, Vernet R, Jannot AS, Neuraz A, Blanco C, et al. Observational study of haloperidol in hospitalized patients with COVID-19. PLoS ONE. 2021;16:e0247122.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.2307/3314608",
"author": "B Efron",
"doi-asserted-by": "publisher",
"first-page": "139",
"journal-title": "Can J Stat",
"key": "2004_CR56",
"unstructured": "Efron B. Nonparametric standard errors and confidence intervals. Can J Stat. 1981;9:139–58.",
"volume": "9",
"year": "1981"
},
{
"key": "2004_CR57",
"unstructured": "Kassambara A, Kosinski M, Biecek P. Survminer: Drawing Survival Curves Using “Ggplot2”. 2020. https://CRAN.R-project.org/package=survminer."
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "publisher",
"key": "2004_CR58",
"unstructured": "Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42. https://doi.org/10.18637/jss.v042.i08."
},
{
"DOI": "10.4103/2229-3485.173773",
"author": "P Ranganathan",
"doi-asserted-by": "publisher",
"first-page": "51",
"journal-title": "Perspect Clin Res",
"key": "2004_CR59",
"unstructured": "Ranganathan P, Pramesh C, Aggarwal R. Common pitfalls in statistical analysis: absolute risk reduction, relative risk reduction, and number needed to treat. Perspect Clin Res. 2016;7:51.",
"volume": "7",
"year": "2016"
},
{
"key": "2004_CR60",
"unstructured": "Yang Z. Nnt: the number needed to treat (NNT) for survival endpoint. 2020. https://CRAN.R-project.org/package=nnt."
},
{
"DOI": "10.1007/978-1-4757-3294-8",
"doi-asserted-by": "crossref",
"key": "2004_CR61",
"unstructured": "Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer; New York, USA; 2000."
},
{
"DOI": "10.1093/biomet/81.3.515",
"author": "PM Grambsch",
"doi-asserted-by": "publisher",
"first-page": "515",
"journal-title": "Biometrika.",
"key": "2004_CR62",
"unstructured": "Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26.",
"volume": "81",
"year": "1994"
},
{
"key": "2004_CR63",
"unstructured": "R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Auckland, New Zealand; 2022. https://www.R-project.org/."
},
{
"DOI": "10.1192/bjo.2021.1053",
"author": "CL Clelland",
"doi-asserted-by": "publisher",
"first-page": "e6",
"journal-title": "BJPsych Open",
"key": "2004_CR64",
"unstructured": "Clelland CL, Ramiah K, Steinberg L, Clelland JD. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych Open. 2021;8:e6.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111437",
"author": "JF Creeden",
"doi-asserted-by": "publisher",
"first-page": "111437",
"journal-title": "Biomed Pharmacother",
"key": "2004_CR65",
"unstructured": "Creeden JF, Imami AS, Eby HM, Gillman C, Becker KN, Reigle J, et al. Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomed Pharmacother. 2021;138:111437.",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(21)00588-X",
"author": "G Reis",
"doi-asserted-by": "publisher",
"first-page": "e333",
"journal-title": "Lancet Glob Health",
"key": "2004_CR66",
"unstructured": "Reis G, Mills E. Fluvoxamine for the treatment of COVID-19—author’s reply. Lancet Glob Health. 2022;10:e333.",
"volume": "10",
"year": "2022"
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41380-023-02004-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cellular and Molecular Neuroscience",
"Psychiatry and Mental health",
"Molecular Biology"
],
"subtitle": [],
"title": "Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: a prospective interventional open-label cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
