Early Fluvoxamine Reduces the Risk for Clinical Deterioration in Symptomatic Outpatients with COVID-19: A Real-World, Retrospective, before–after Analysis
et al., Microorganisms, doi:10.3390/microorganisms11082073, Aug 2023
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
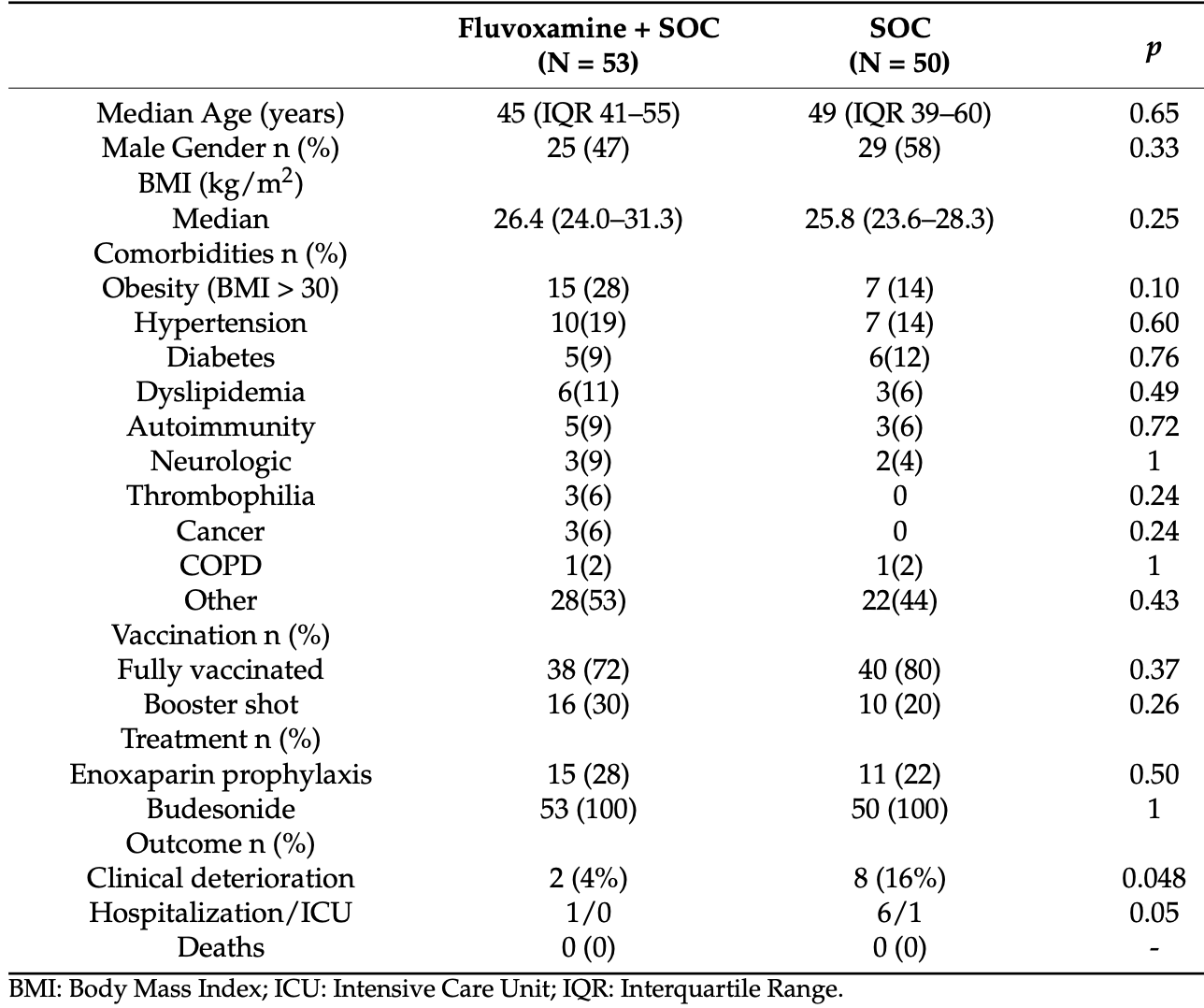
Retrospective 103 outpatients in Greece, showing lower risk of progression with fluvoxamine 100mg bid for 10 days. 2 patients (4%) in the fluvoxamine group had clinical deterioration compared to 8 patients (16%) in the standard care group (p<0.05). After adjusting for confounders, fluvoxamine was associated with a lower risk of clinical deterioration (adjusted OR 0.12, p=0.02). Fluvoxamine was also associated with improved lymphocyte count. Control patients were during Sep-Nov 2021, and treatment patients Nov-Dec 2021, introducing potential confounding by time due to changes in variants, although the change in risk during this period is expected to be relatively low.
|
risk of ICU admission, 67.3% lower, RR 0.33, p = 0.49, treatment 0 of 53 (0.0%), control 1 of 50 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 84.3% lower, RR 0.16, p = 0.06, treatment 1 of 53 (1.9%), control 6 of 50 (12.0%), NNT 9.9.
|
|
risk of progression, 86.0% lower, RR 0.14, p = 0.02, treatment 2 of 53 (3.8%), control 8 of 50 (16.0%), NNT 8.2, adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tsiakalos et al., 12 Aug 2023, retrospective, Greece, peer-reviewed, 5 authors, study period 1 September, 2021 - 31 December, 2021.
Early Fluvoxamine Reduces the Risk for Clinical Deterioration in Symptomatic Outpatients with COVID-19: A Real-World, Retrospective, before-after Analysis
doi:10.3390/microorganisms11082073
Fluvoxamine, a selective serotonin reuptake inhibitor with anti-inflammatory properties, has gained attention as a repurposed drug to treat COVID-19. We aimed to explore the potential benefit of fluvoxamine on outpatients with early SARS-CoV-2 infection. We performed a retrospective study of fluvoxamine adult outpatients with symptomatic COVID-19 disease of early onset (<5 days), in the context of an infectious diseases private practice, between September-December 2021, in Greece. Patients with disease duration ≥5 days, dyspnea and/or hypoxemia with oxygen saturation <94% in room air and pregnancy were excluded from the analysis. In total, 103 patients, 54 males/49 females with a median age of 47 years (39-56), were included in this study. Patient characteristics were balanced before and after the introduction of fluvoxamine. Two patients in the fluvoxamine arm (3.8%; 95% CI 0.4-13) had clinical deterioration compared to 8 patients in the standard of care group (16%; 95% CI 7.2-29.1, p < 0.04). After controlling for age, sex, body mass index > 30 and vaccination status, fluvoxamine was independently associated with a lower risk of clinical deterioration (adj. OR 0.12; 95% CI 0.02-0.70, p < 0.02). Adding on fluvoxamine to treatment for early symptomatic COVID-19 patients may protect them from clinical deterioration and hospitalization, and it is an appealing low-cost, low-toxicity option in the community setting and warrants further investigation.
Author Contributions: A.T. and P.D.Z. conceived the idea, A.T. and P.D.Z. collected and analyzed data, A.T., P.D.Z., K.A., E.P. and G.S. wrote the manuscript, K.A. and P.D.Z. critically corrected the manuscript, A.T. oversaw the study. All authors have read and agreed to the published version of the manuscript. Funding: This research received no external funding.
Conflicts of Interest: The authors declare no conflict of interest.
References
Adnot, Houssaini, Abid, Marcos, Amsellem, Serotonin transporter and serotonin receptors, Handb. Exp. Pharmacol, doi:10.1007/978-3-642-38664-0_15
Arulanandam, Beladi, Chakrabarti, Obesity and COVID-19 mortality are correlated, Sci. Rep, doi:10.1038/s41598-023-33093-3
Berwanger, Antithrombotic Therapy for Outpatients with COVID-19: Implications for Clinical Practice and Future Research, JAMA, doi:10.1001/jama.2021.17460
Boretti, Effectiveness of fluvoxamine at preventing COVID-19 infection from turning severe, Eur. Neuropsychopharmacol, doi:10.1016/j.euroneuro.2022.12.001
Bramante, Buse, Liebovitz, Nicklas, Puskarich et al., Outpatient treatment of COVID-19 and incidence of post-COVID-19
Bramante, Huling, Tignanelli, Buse, Liebovitz et al., Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2201662
Calusic, Marcec, Luksa, Jurkovic, Kovac et al., Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls, Br. J. Clin. Pharmacol, doi:10.1111/bcp.15126
Carpinteiro, Edwards, Hoffmann, Kochs, Gripp et al., Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells, Cell Rep. Med, doi:10.1016/j.xcrm.2020.100142
Chakraborty, Sharma, Bhattacharya, Agoramoorthy, Lee, The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies, Front. Pharmacol, doi:10.3389/fphar.2021.704205
Chalmers, Chotirmall, Rewiring the Immune Response in COVID-19, Am. J. Respir. Crit. Care Med
Coveney, Firthlogit, Stata Module to Calculate Bias Reduction in Logistic Regression
Deng, Rayner, Ramaraju, Abbas, Garcia et al., Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: A systematic review and meta-analysis, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2023.01.010
Diao, Wang, Tan, Chen, Liu et al., Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19), Front. Immunol, doi:10.3389/fimmu.2020.00827
Dicker, Bettini, Farpour-Lambert, Fruhbeck, Golan et al., Obesity and COVID-19: The Two Sides of the Coin, Obes. Facts, doi:10.1159/000510005
Drewry, Samra, Skrupky, Fuller, Compton et al., Persistent lymphopenia after diagnosis of sepsis predicts mortality, Shock, doi:10.1097/SHK.0000000000000234
Ebell, Inhaled Budesonide Reduces the Risk of Emergency Department Evaluation or Hospitalization in Early COVID-19, Am. Fam. Physician
Fergal, Gilmar, Kristian, Jamie, Christina et al., TOGETHER Investigators. Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model. medRxiv 2021, doi:10.1101/2021.12.23.21268352
Fico, Isayeva, De Prisco, Oliva, Sole et al., Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis, Eur. Neuropsychopharmacol, doi:10.1016/j.euroneuro.2022.10.004
Finley, Is Fluvoxamine the Covid Drug We've Been Waiting for? Available online
Firth, Bias reduction of maximum likelihood estimates, Biometrika, doi:10.1093/biomet/80.1.27
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Hashimoto, Suzuki, Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: A historical review, Mol. Psychiatry, doi:10.1038/s41380-021-01432-3
He, Zhao, Dong, Zhuang, Song et al., Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets, Int. J. Infect. Dis, doi:10.1016/j.ijid.2004.07.014
Heinze, Schemper, A solution to the problem of separation in logistic regression, Stat. Med, doi:10.1002/sim.1047
Hoertel, Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality among Patients with COVID-19?, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.36510
Huang, Pranata, Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis, J. Intensive Care, doi:10.1186/s40560-020-00453-4
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Lancet, doi:10.1016/S0140-6736(20)30183-5
Kirenga, Mugenyi, Sanchez-Rico, Kyobe, Muttamba et al., Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: A prospective interventional open-label cohort study, Mol. Psychiatry, doi:10.1038/s41380-023-02004-3
Lee, Vigod, Bortolussi-Courval, Hanula, Boulware et al., Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.6269
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients with Symptomatic COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.22760
Marcec, Dodig, Likic, A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference, J. Infect, doi:10.1016/j.jinf.2022.11.011
Marzolini, Marra, Boyle, Khoo, Back, Fluvoxamine for the treatment of COVID-19, Lancet Glob Health, doi:10.1016/S2214-109X(21)00592-1
Maxwell, Sanders, Sabot, Hachem, Llanos-Cuentas et al., COVID-19 Therapeutics for Low-and Middle-Income Countries: A Review of Candidate Agents with Potential for Near-Term Use and Impact, Am. J. Trop. Med. Hyg, doi:10.4269/ajtmh.21-0200
Mccarthy, Naggie, Boulware, Lindsell, Stewart et al., Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients with Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.24100
Mills, Reis, Wilson, Thorlund, Forrest et al., Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model, Am. J. Trop. Med. Hyg. 2023, doi:10.4269/ajtmh.22-0106
Niu, Sareli, Mayer, Visbal, Sareli, Lymphopenia as a Predictor for Adverse Clinical Outcomes in Hospitalized Patients with COVID-19: A Single Center Retrospective Study of 4485 Cases, J. Clin. Med, doi:10.3390/jcm11030700
Oskotsky, Maric, Tang, Oskotsky, Wong et al., Mortality Risk among Patients with COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.33090
Pericat, Leon-Icaza, Sanchez Rico, Muhle, Zoicas et al., Antiviral and Anti-Inflammatory Activities of Fluoxetine in a SARS-CoV-2 Infection Mouse Model, Int. J. Mol. Sci, doi:10.3390/ijms232113623
Pontelli, Castro, Martins, La Serra, Veras et al., SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients, J. Mol. Cell Biol, doi:10.1093/jmcb/mjac021
Prices, None
Rafiee, Hajhashemi, Javanmard, Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat, Iran. J. Basic. Med. Sci
Rahman, Mahi, Melamed, Alam, Effects of Antidepressants on COVID-19 Outcomes: Retrospective Study on Large-Scale Electronic Health Record Data. Interact, J. Med. Res, doi:10.2196/39455
Ramakrishnan, Nicolau, Jr, Langford, Mahdi et al., Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00160-0
Ratajczak, Kucia, SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine "storm" and risk factor for damage of hematopoietic stem cells, Leukemia, doi:10.1038/s41375-020-0887-9
Reis, Dos, Moreira-Silva, Silva, Thabane et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial, Lancet Glob. Health, doi:10.1016/S2214-109X(21)00448-4
Reis, Dos, Silva, Medeiros Silva, Thabane et al., Oral Fluvoxamine with Inhaled Budesonide for Treatment of Early-Onset COVID-19: A Randomized Platform Trial, Ann. Intern. Med, doi:10.7326/M22-3305
Reis, Mills, Fluvoxamine for the treatment of COVID-19-Author's reply, Lancet Glob. Health, doi:10.1016/S2214-109X(21)00588-X
Rosen, Seki, Fernández-Castañeda, Beiter, Eccles et al., Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis, Sci. Transl. Med
Russell, Lone, Baillie, Comorbidities, multimorbidity and COVID-19, Nat. Med. 2023, doi:10.1038/s41591-022-02156-9
Sattar, Mcinnes, Mcmurray, Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms, Circulation, doi:10.1161/CIRCULATIONAHA.120.047659
Sawadogo, Tsegaye, Gizaw, Adera, Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis, BMJ Nutr. Prev. Health, doi:10.1136/bmjnph-2021-000375
Talasaz, Sadeghipour, Kakavand, Aghakouchakzadeh, Kordzadeh-Kermani et al., Recent Randomized Trials of Antithrombotic Therapy for Patients with COVID-19: JACC State-of-the-Art Review, J. Am. Coll. Cardiol, doi:10.1016/j.jacc.2021.02.035
Usher, The global COVID-19 treatment divide, Lancet, doi:10.1016/S0140-6736(22)00372-5
Van Harten, Overview of the pharmacokinetics of fluvoxamine, Clin. Pharmacokinet, doi:10.2165/00003088-199500291-00003
Varghese, Sandmann, Ochs, Schrempf, Frommel et al., Persistent symptoms and lab abnormalities in patients who recovered from COVID-19, Sci. Rep, doi:10.1038/s41598-021-91270-8
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis, Ann. Med, doi:10.1080/07853890.2022.2034936
Wu, Joyal-Desmarais, Ribeiro, Vieira, Stojanovic et al., Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, Lancet Respir. Med, doi:10.1016/S2213-2600(23)00015-2
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
Zhao, Meng, Kumar, Wu, Huang et al., Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.04.086
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan
Zimniak, Kirschner, Hilpert, Geiger, Danov et al., The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci, doi:10.1038/s41598-021-85049-0
DOI record:
{
"DOI": "10.3390/microorganisms11082073",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms11082073",
"abstract": "<jats:p>Fluvoxamine, a selective serotonin reuptake inhibitor with anti-inflammatory properties, has gained attention as a repurposed drug to treat COVID-19. We aimed to explore the potential benefit of fluvoxamine on outpatients with early SARS-CoV-2 infection. We performed a retrospective study of fluvoxamine adult outpatients with symptomatic COVID-19 disease of early onset (<5 days), in the context of an infectious diseases private practice, between September–December 2021, in Greece. Patients with disease duration ≥5 days, dyspnea and/or hypoxemia with oxygen saturation <94% in room air and pregnancy were excluded from the analysis. In total, 103 patients, 54 males/49 females with a median age of 47 years (39–56), were included in this study. Patient characteristics were balanced before and after the introduction of fluvoxamine. Two patients in the fluvoxamine arm (3.8%; 95% CI 0.4–13) had clinical deterioration compared to 8 patients in the standard of care group (16%; 95% CI 7.2–29.1, p < 0.04). After controlling for age, sex, body mass index > 30 and vaccination status, fluvoxamine was independently associated with a lower risk of clinical deterioration (adj. OR 0.12; 95% CI 0.02–0.70, p < 0.02). Adding on fluvoxamine to treatment for early symptomatic COVID-19 patients may protect them from clinical deterioration and hospitalization, and it is an appealing low-cost, low-toxicity option in the community setting and warrants further investigation.</jats:p>",
"alternative-id": [
"microorganisms11082073"
],
"author": [
{
"affiliation": [
{
"name": "Leto General, Maternity & Gynecology Clinic, 11524 Athens, Greece"
}
],
"family": "Tsiakalos",
"given": "Aristotelis",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine, Brown University, Providence, RI 02912, USA"
}
],
"family": "Ziakas",
"given": "Panayiotis D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dept of Internal Medicine and Infectious Diseases, Medical School, University General Hospital of Patras, University of Patras, 26504 Rio, Greece"
}
],
"family": "Polyzou",
"given": "Eleni",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7963-1865",
"affiliation": [
{
"name": "Dept of Internal Medicine and Infectious Diseases, Medical School, University General Hospital of Patras, University of Patras, 26504 Rio, Greece"
}
],
"authenticated-orcid": false,
"family": "Schinas",
"given": "Georgios",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4289-9494",
"affiliation": [
{
"name": "Dept of Internal Medicine and Infectious Diseases, Medical School, University General Hospital of Patras, University of Patras, 26504 Rio, Greece"
}
],
"authenticated-orcid": false,
"family": "Akinosoglou",
"given": "Karolina",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
14
]
],
"date-time": "2023-08-14T14:20:14Z",
"timestamp": 1692022814000
},
"deposited": {
"date-parts": [
[
2023,
8,
14
]
],
"date-time": "2023-08-14T15:40:36Z",
"timestamp": 1692027636000
},
"indexed": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T05:22:13Z",
"timestamp": 1708406533337
},
"is-referenced-by-count": 2,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
8,
12
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
12
]
],
"date-time": "2023-08-12T00:00:00Z",
"timestamp": 1691798400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/11/8/2073/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2073",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
8,
12
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
12
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1164/rccm.202007-2934ED",
"article-title": "Rewiring the Immune Response in COVID-19",
"author": "Chalmers",
"doi-asserted-by": "crossref",
"first-page": "784",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_1",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1038/s41591-022-02156-9",
"article-title": "Comorbidities, multimorbidity and COVID-19",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "334",
"journal-title": "Nat. Med.",
"key": "ref_2",
"volume": "29",
"year": "2023"
},
{
"key": "ref_3",
"unstructured": "National Institutes of Health (2023, April 15). COVID-19 TREATMENT GUIDELINES, Available online: https://www.covid19treatmentguidelines.nih.gov/."
},
{
"DOI": "10.1016/S0140-6736(22)00372-5",
"article-title": "The global COVID-19 treatment divide",
"author": "Usher",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Lancet",
"key": "ref_4",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.4269/ajtmh.21-0200",
"article-title": "COVID-19 Therapeutics for Low- and Middle-Income Countries: A Review of Candidate Agents with Potential for Near-Term Use and Impact",
"author": "Maxwell",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "Am. J. Trop. Med. Hyg.",
"key": "ref_5",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00242-7",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Lancet Commission on COVID-19 Vaccines and Therapeutics Task Force Members (2021). Urgent needs of low-income and middle-income countries for COVID-19 vaccines and therapeutics. Lancet, 397, 562–564."
},
{
"DOI": "10.3389/fphar.2021.704205",
"article-title": "The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies",
"author": "Chakraborty",
"doi-asserted-by": "crossref",
"first-page": "704205",
"journal-title": "Front. Pharmacol.",
"key": "ref_7",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"article-title": "Mortality Risk among Patients with COVID-19 Prescribed Selective Serotonin Reuptake Inhibitor Antidepressants",
"author": "Oskotsky",
"doi-asserted-by": "crossref",
"first-page": "e2133090",
"journal-title": "JAMA Netw. Open",
"key": "ref_8",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-85049-0",
"article-title": "The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue",
"author": "Zimniak",
"doi-asserted-by": "crossref",
"first-page": "5890",
"journal-title": "Sci. Rep.",
"key": "ref_9",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.aau5266",
"article-title": "Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis",
"author": "Rosen",
"doi-asserted-by": "crossref",
"first-page": "eaau5266",
"journal-title": "Sci. Transl. Med.",
"key": "ref_10",
"volume": "11",
"year": "2019"
},
{
"article-title": "Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat",
"author": "Rafiee",
"first-page": "977",
"journal-title": "Iran. J. Basic. Med. Sci.",
"key": "ref_11",
"volume": "19",
"year": "2016"
},
{
"DOI": "10.1038/s41380-021-01432-3",
"article-title": "Mechanisms of action of fluvoxamine for COVID-19: A historical review",
"author": "Hashimoto",
"doi-asserted-by": "crossref",
"first-page": "1898",
"journal-title": "Mol. Psychiatry",
"key": "ref_12",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.3390/ijms232113623",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Pericat, D., Leon-Icaza, S.A., Sanchez Rico, M., Muhle, C., Zoicas, I., Schumacher, F., Planes, R., Mazars, R., Gros, G., and Carpinteiro, A. (2022). Antiviral and Anti-Inflammatory Activities of Fluoxetine in a SARS-CoV-2 Infection Mouse Model. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1007/978-3-642-38664-0_15",
"article-title": "Serotonin transporter and serotonin receptors",
"author": "Adnot",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Handb. Exp. Pharmacol.",
"key": "ref_14",
"volume": "218",
"year": "2013"
},
{
"DOI": "10.1038/s41380-023-02004-3",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Kirenga, B.J., Mugenyi, L., Sanchez-Rico, M., Kyobe, H., Muttamba, W., Mugume, R., Mwesigwa, E., Kalimo, E., Nyombi, V., and Segawa, I. (2023). Association of fluvoxamine with mortality and symptom resolution among inpatients with COVID-19 in Uganda: A prospective interventional open-label cohort study. Mol. Psychiatry, epub ahead of print."
},
{
"DOI": "10.1016/j.xcrm.2020.100142",
"article-title": "Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells",
"author": "Carpinteiro",
"doi-asserted-by": "crossref",
"first-page": "100142",
"journal-title": "Cell Rep. Med.",
"key": "ref_16",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs Placebo and Clinical Deterioration in Outpatients with Symptomatic COVID-19: A Randomized Clinical Trial",
"author": "Lenze",
"doi-asserted-by": "crossref",
"first-page": "2292",
"journal-title": "JAMA",
"key": "ref_17",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: The TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"journal-title": "Lancet Glob. Health",
"key": "ref_18",
"volume": "10",
"year": "2022"
},
{
"key": "ref_19",
"unstructured": "Finley, A. (2022, January 14). Is Fluvoxamine the Covid Drug We’ve Been Waiting for?. Available online: https://www.wsj.com/articles/is-fluvoxamine-the-covid-miracle-drug-we-have-been-waiting-for-oral-pill-cheap-hospitalization-11640726605."
},
{
"key": "ref_20",
"unstructured": "Fergal, P.M., Gilmar, R., Kristian, T., Jamie, I.F., Christina, M.G., David, R.B., Edward, J.M., and TOGETHER Investigators (2021). Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model. medRxiv."
},
{
"key": "ref_21",
"unstructured": "National Public Health Organization (2022, August 01). Guidelines for Healthcare Professionals and Areas of Healthcare Services. Therapeutic Algorithm for Adult Non-Hospitalized Patients with COVID-19, Available online: https://eody.gov.gr/neos-koronaios-covid-19/."
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Lancet",
"key": "ref_22",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Lancet Respir. Med.",
"key": "ref_23",
"volume": "9",
"year": "2021"
},
{
"article-title": "Inhaled Budesonide Reduces the Risk of Emergency Department Evaluation or Hospitalization in Early COVID-19",
"author": "Ebell",
"first-page": "207",
"journal-title": "Am. Fam. Physician",
"key": "ref_24",
"volume": "104",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_25",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "ref_26",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "ref_27",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "ref_28",
"volume": "323",
"year": "2020"
},
{
"key": "ref_29",
"unstructured": "Coveney, J. (2023, April 15). FIRTHLOGIT: Stata Module to Calculate Bias Reduction in Logistic Regression. Available online: https://EconPapers.repec.org/RePEc:boc:bocode:s456948."
},
{
"DOI": "10.1016/S2214-109X(21)00588-X",
"article-title": "Fluvoxamine for the treatment of COVID-19—Author’s reply",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e333",
"journal-title": "Lancet Glob. Health",
"key": "ref_30",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.7326/M22-3305",
"article-title": "Oral Fluvoxamine with Inhaled Budesonide for Treatment of Early-Onset COVID-19: A Randomized Platform Trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "Ann. Intern. Med.",
"key": "ref_31",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2021.36510",
"article-title": "Do the Selective Serotonin Reuptake Inhibitor Antidepressants Fluoxetine and Fluvoxamine Reduce Mortality among Patients with COVID-19?",
"author": "Hoertel",
"doi-asserted-by": "crossref",
"first-page": "e2136510",
"journal-title": "JAMA Netw. Open",
"key": "ref_32",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.euroneuro.2022.10.004",
"article-title": "Psychotropic drug repurposing for COVID-19: A Systematic Review and Meta-Analysis",
"author": "Fico",
"doi-asserted-by": "crossref",
"first-page": "30",
"journal-title": "Eur. Neuropsychopharmacol.",
"key": "ref_33",
"volume": "66",
"year": "2023"
},
{
"DOI": "10.1016/j.euroneuro.2022.12.001",
"article-title": "Effectiveness of fluvoxamine at preventing COVID-19 infection from turning severe",
"author": "Boretti",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Eur. Neuropsychopharmacol.",
"key": "ref_34",
"volume": "67",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2022.6269",
"article-title": "Fluvoxamine for Outpatient Management of COVID-19 to Prevent Hospitalization: A Systematic Review and Meta-analysis",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e226269",
"journal-title": "JAMA Netw. Open",
"key": "ref_35",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2022.11.011",
"article-title": "A meta-analysis regarding fluvoxamine and hospitalization risk of COVID-19 patients: TOGETHER making a difference",
"author": "Marcec",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "J. Infect.",
"key": "ref_36",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann. Med.",
"key": "ref_37",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2023.01.010",
"article-title": "Efficacy and safety of selective serotonin reuptake inhibitors in COVID-19 management: A systematic review and meta-analysis",
"author": "Deng",
"doi-asserted-by": "crossref",
"first-page": "578",
"journal-title": "Clin. Microbiol. Infect.",
"key": "ref_38",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15126",
"article-title": "Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls",
"author": "Calusic",
"doi-asserted-by": "crossref",
"first-page": "2065",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_39",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.2196/39455",
"article-title": "Effects of Antidepressants on COVID-19 Outcomes: Retrospective Study on Large-Scale Electronic Health Record Data",
"author": "Rahman",
"doi-asserted-by": "crossref",
"first-page": "e39455",
"journal-title": "Interact. J. Med. Res.",
"key": "ref_40",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for COVID-19",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N. Engl. J. Med.",
"key": "ref_41",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Bramante, C.T., Buse, J.B., Liebovitz, D.M., Nicklas, J.M., Puskarich, M.A., Cohen, K., Belani, H.K., Anderson, B.J., Huling, J.D., and Tignanelli, C.J. (2023). Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect. Dis., epub ahead of print."
},
{
"DOI": "10.1001/jama.2022.24100",
"article-title": "Effect of Fluvoxamine vs Placebo on Time to Sustained Recovery in Outpatients with Mild to Moderate COVID-19: A Randomized Clinical Trial",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "296",
"journal-title": "JAMA",
"key": "ref_43",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1016/S2214-109X(21)00592-1",
"article-title": "Fluvoxamine for the treatment of COVID-19",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "e331",
"journal-title": "Lancet Glob Health",
"key": "ref_44",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.2165/00003088-199500291-00003",
"article-title": "Overview of the pharmacokinetics of fluvoxamine",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Clin. Pharmacokinet.",
"key": "ref_45",
"volume": "29",
"year": "1995"
},
{
"key": "ref_46",
"unstructured": "(2023, April 15). Fluvoxamine Prices GoodRx. Available online: https://www.ramq.gouv.qc.ca/en/media/12091."
},
{
"DOI": "10.4269/ajtmh.22-0106",
"article-title": "Early Treatment with Fluvoxamine among Patients with COVID-19: A Cost-Consequence Model",
"author": "Mills",
"doi-asserted-by": "crossref",
"first-page": "101",
"journal-title": "Am. J. Trop. Med. Hyg.",
"key": "ref_47",
"volume": "108",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-33093-3",
"article-title": "Obesity and COVID-19 mortality are correlated",
"author": "Arulanandam",
"doi-asserted-by": "crossref",
"first-page": "5895",
"journal-title": "Sci. Rep.",
"key": "ref_48",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1136/bmjnph-2021-000375",
"article-title": "Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis",
"author": "Sawadogo",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "BMJ Nutr. Prev. Health",
"key": "ref_49",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.047659",
"article-title": "Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms",
"author": "Sattar",
"doi-asserted-by": "crossref",
"first-page": "4",
"journal-title": "Circulation",
"key": "ref_50",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1159/000510005",
"article-title": "Obesity and COVID-19: The Two Sides of the Coin",
"author": "Dicker",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Obes. Facts",
"key": "ref_51",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(23)00015-2",
"article-title": "Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "439",
"journal-title": "Lancet Respir. Med.",
"key": "ref_52",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1186/s40560-020-00453-4",
"article-title": "Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "36",
"journal-title": "J. Intensive Care",
"key": "ref_53",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.04.086",
"article-title": "Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_54",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.3390/jcm11030700",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Niu, J., Sareli, C., Mayer, D., Visbal, A., and Sareli, A. (2022). Lymphopenia as a Predictor for Adverse Clinical Outcomes in Hospitalized Patients with COVID-19: A Single Center Retrospective Study of 4485 Cases. J. Clin. Med., 11."
},
{
"DOI": "10.3389/fimmu.2020.00827",
"article-title": "Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19)",
"author": "Diao",
"doi-asserted-by": "crossref",
"first-page": "827",
"journal-title": "Front. Immunol.",
"key": "ref_56",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/jmcb/mjac021",
"article-title": "SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients",
"author": "Pontelli",
"doi-asserted-by": "crossref",
"first-page": "mjac021",
"journal-title": "J. Mol. Cell Biol.",
"key": "ref_57",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41375-020-0887-9",
"article-title": "SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells",
"author": "Ratajczak",
"doi-asserted-by": "crossref",
"first-page": "1726",
"journal-title": "Leukemia",
"key": "ref_58",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2004.07.014",
"article-title": "Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_59",
"volume": "9",
"year": "2005"
},
{
"DOI": "10.1038/s41598-021-91270-8",
"article-title": "Persistent symptoms and lab abnormalities in patients who recovered from COVID-19",
"author": "Varghese",
"doi-asserted-by": "crossref",
"first-page": "12775",
"journal-title": "Sci. Rep.",
"key": "ref_60",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1097/SHK.0000000000000234",
"article-title": "Persistent lymphopenia after diagnosis of sepsis predicts mortality",
"author": "Drewry",
"doi-asserted-by": "crossref",
"first-page": "383",
"journal-title": "Shock",
"key": "ref_61",
"volume": "42",
"year": "2014"
},
{
"DOI": "10.1093/biomet/80.1.27",
"article-title": "Bias reduction of maximum likelihood estimates",
"author": "Firth",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Biometrika",
"key": "ref_62",
"volume": "80",
"year": "1993"
},
{
"DOI": "10.1002/sim.1047",
"article-title": "A solution to the problem of separation in logistic regression",
"author": "Heinze",
"doi-asserted-by": "crossref",
"first-page": "2409",
"journal-title": "Stat. Med.",
"key": "ref_63",
"volume": "21",
"year": "2002"
},
{
"DOI": "10.1001/jama.2021.17460",
"article-title": "Antithrombotic Therapy for Outpatients with COVID-19: Implications for Clinical Practice and Future Research",
"author": "Berwanger",
"doi-asserted-by": "crossref",
"first-page": "1685",
"journal-title": "JAMA",
"key": "ref_64",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/j.jacc.2021.02.035",
"article-title": "Recent Randomized Trials of Antithrombotic Therapy for Patients with COVID-19: JACC State-of-the-Art Review",
"author": "Talasaz",
"doi-asserted-by": "crossref",
"first-page": "1903",
"journal-title": "J. Am. Coll. Cardiol.",
"key": "ref_65",
"volume": "77",
"year": "2021"
}
],
"reference-count": 65,
"references-count": 65,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/11/8/2073"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Early Fluvoxamine Reduces the Risk for Clinical Deterioration in Symptomatic Outpatients with COVID-19: A Real-World, Retrospective, before–after Analysis",
"type": "journal-article",
"volume": "11"
}
