Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial
et al., Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00160-0, STOIC, NCT04416399, Feb 2021 (preprint)
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
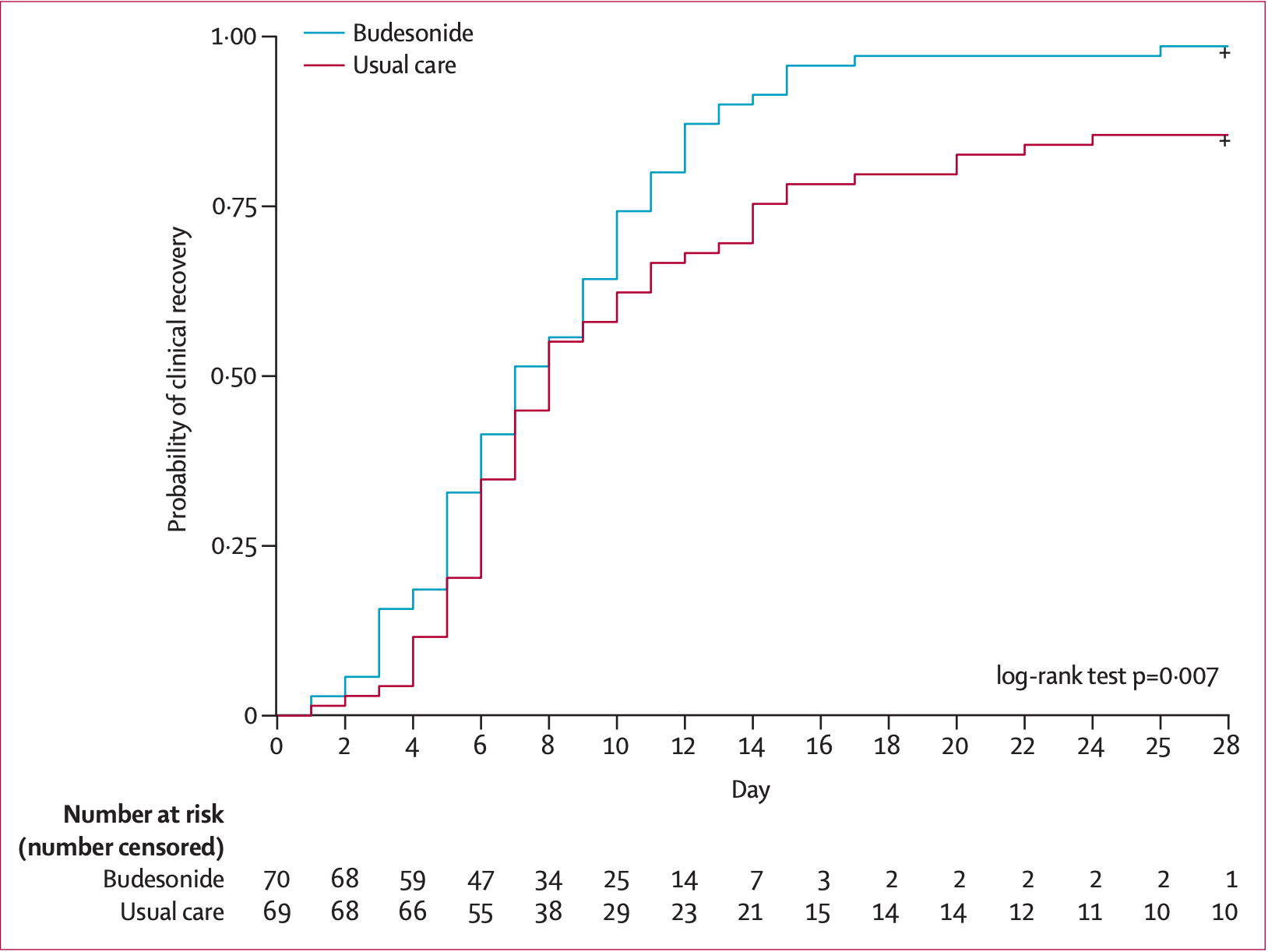
RCT with 73 budesonide patients and 73 control patients, showing significantly lower combined risk of an ER visit or hospitalization, and lower risk of no recovery at day 14.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization/ER, 81.8% lower, RR 0.18, p = 0.02, treatment 2 of 73 (2.7%), control 11 of 73 (15.1%), NNT 8.1, ITT.
|
|
risk of hospitalization/ER, 90.1% lower, RR 0.10, p = 0.004, treatment 1 of 70 (1.4%), control 10 of 69 (14.5%), NNT 7.7, PP.
|
|
risk of no recovery, 67.1% lower, RR 0.33, p = 0.003, treatment 7 of 70 (10.0%), control 21 of 69 (30.4%), NNT 4.9, PP, day 14.
|
|
recovery time, 12.5% lower, relative time 0.88, treatment 70, control 69, PP.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ramakrishnan et al., 8 Feb 2021, Randomized Controlled Trial, United Kingdom, peer-reviewed, 24 authors, study period 16 July, 2020 - 9 December, 2020, average treatment delay 3.0 days, trial NCT04416399 (history) (STOIC).
Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00160-0
Background Multiple early reports of patients admitted to hospital with COVID-19 showed that patients with chronic respiratory disease were significantly under-represented in these cohorts. We hypothesised that the widespread use of inhaled glucocorticoids among these patients was responsible for this finding, and tested if inhaled glucocorticoids would be an effective treatment for early COVID-19.
Methods We performed an open-label, parallel-group, phase 2, randomised controlled trial (Steroids in COVID-19; STOIC) of inhaled budesonide, compared with usual care, in adults within 7 days of the onset of mild COVID-19 symptoms. The trial was done in the community in Oxfordshire, UK. Participants were randomly assigned to inhaled budsonide or usual care stratified for age (≤40 years or >40 years), sex (male or female), and number of comorbidities (≤1 and ≥2). Randomisation was done using random sequence generation in block randomisation in a 1:1 ratio. Budesonide dry powder was delivered using a turbohaler at a dose of 400 μg per actuation. Participants were asked to take two inhalations twice a day until symptom resolution. The primary endpoint was COVID-19-related urgent care visit, including emergency department assessment or hospitalisation, analysed for both the per-protocol and intentionto-treat (ITT) populations. The secondary outcomes were self-reported clinical recovery (symptom resolution), viral symptoms measured using the Common Cold Questionnare (CCQ) and the InFLUenza Patient Reported Outcome Questionnaire (FLUPro), body temperature, blood oxygen saturations, and SARS-CoV-2 viral load. The trial was stopped early after independent statistical review concluded that study outcome would not change with further participant enrolment. This trial is registered with ClinicalTrials.gov, NCT04416399.
Findings From July 16 to Dec 9, 2020, 167 participants were recruited and assessed for eligibility. 21 did not meet eligibility criteria and were excluded. 146 participants were randomly assigned-73 to usual care and 73 to budesonide. For the per-protocol population (n=139), the primary outcome occurred in ten (14%) of 70 participants in the usual care group and one (1%) of 69 participants in the budesonide group (difference in proportions 0•131, 95% CI 0•043 to 0•218; p=0•004). For the ITT population, the primary outcome occurred in 11 (15%) participants in the usual care group and two (3%) participants in the budesonide group (difference in proportions 0•123, 95% CI 0•033 to 0•213; p=0•009). The number needed to treat with inhaled budesonide to reduce COVID-19 deterioration was eight. Clinical recovery was 1 day shorter in the budesonide group compared with the usual care group (median 7 days [95% CI 6 to 9] in the budesonide group vs 8 days [7 to 11] in the usual care group; log-rank test p=0•007). The mean proportion of days with a fever in the first 14 days was lower in the budesonide group (2%, SD 6) than the usual care group (8%, SD..
References
Bloom, Drake, Docherty, Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK, Lancet Respir Med, doi:10.1016/S2213-2600(21)00013-8
Callaway, Could new COVID variants undermine vaccines? Labs scramble to find out, Nature
Choi, Jung, Yoon, Inhaled corticosteroids and COVID-19 risk and mortality: a nationwide cohort study, J Clin Med
Edejer, Hanssen, Mirelman, Projected health-care resource needs for an effective response to COVID-19 in 73 low-income and middle-income countries: a modelling study, Lancet Glob Health
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Heffler, Detoraki, Contoli, COVID-19 in Severe Asthma Network in Italy (SANI) patients: Clinical features, impact of comorbidities and treatments, Allergy
Ho, Howell, Rogers, Narasimhan, Verma et al., The relationship between asthma, eosinophilia, and outcomes in COVID-19 infection, Ann Allergy Asthma Immunol
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Kim, Song, Ahn, Antiviral and anti-inflammatory activity of budesonide against human rhinovirus infection mediated via autophagy activation, Antiviral Res
Lauer, Grantz, Bi, The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application, Ann Intern Med
Marcello, Dolle, Grami, Characteristics and outcomes of COVID-19 patients in New York City's public hospital system, PLoS One
Matsuyama, Kawase, Nao, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol
Murthy, Leligdowicz, Adhikari, Intensive care unit capacity in low-income countries: a systematic review, PLoS One
Nguyen-Van-Tam, Openshaw, Hashim, Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009), Thorax
Nicolau, Bafadhel, Inhaled corticosteroids in virus pandemics: a treatment for COVID-19?, Lancet Respir Med
Perchetti, Nalla, Huang, Validation of SARS-CoV-2 detection across multiple specimen types, J Clin Virol
Perego, Callard, Stras, Melville-Jóhannesson, Pope et al., Why the patient-made term 'long COVID' is needed, Wellcome Open Res
Peters, Sajuthi, Deford, COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids, Am J Respir Crit Care Med
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Powell, Smart, Wood, Validity of the common cold questionnaire (CCQ) in asthma exacerbations, PLoS One
Powers, Bacci, Leidy, Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI), PLoS One
Reddel, Jenkins, Quirce, Effect of different asthma treatments on risk of cold-related exacerbations, Eur Respir J
Roozbeh, Saeedi, Alizadeh-Navaei, Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a doubleblind, randomized controlled trial, J Antimicrob Chemother
Skevaki, Karsonova, Karaulov, Xie, Renz, Asthmaassociated risk for COVID-19 development, J Allergy Clin Immunol
Thorlund, Dron, Park, Hsu, Forrest et al., A realtime dashboard of clinical trials for COVID-19, Lancet Digit Health
Tyson, Pedroza, Wallace, 'angio, Bell et al., Stopping guidelines for an effectiveness trial: what should the protocol specify?, Trials
Venarske, Busse, Griffin, The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking, J Infect Dis
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00160-0",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00160-0",
"alternative-id": [
"S2213260021001600"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00160-0"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(21)00171-5"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Ramakrishnan",
"given": "Sanjay",
"sequence": "first"
},
{
"affiliation": [],
"family": "Nicolau",
"given": "Dan V",
"sequence": "additional",
"suffix": "Jr"
},
{
"affiliation": [],
"family": "Langford",
"given": "Beverly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahdi",
"given": "Mahdi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeffers",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mwasuku",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krassowska",
"given": "Karolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binnian",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Glover",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bright",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cane",
"given": "Jennifer L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halner",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matthews",
"given": "Philippa C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donnelly",
"given": "Louise E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simpson",
"given": "Jodie L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baker",
"given": "Jonathan R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fadai",
"given": "Nabil T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peterson",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bengtsson",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barnes",
"given": "Peter J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russell",
"given": "Richard E K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bafadhel",
"given": "Mona",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
4,
9
]
],
"date-time": "2021-04-09T22:33:55Z",
"timestamp": 1618007635000
},
"deposited": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T06:43:08Z",
"timestamp": 1683009788000
},
"funder": [
{
"DOI": "10.13039/100004325",
"doi-asserted-by": "publisher",
"name": "AstraZeneca PLC"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T13:56:27Z",
"timestamp": 1712670987178
},
"is-referenced-by-count": 267,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
7
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021001600?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021001600?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "763-772",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00160-0_bib1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00160-0_bib2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.7326/M20-0504",
"article-title": "The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application",
"author": "Lauer",
"doi-asserted-by": "crossref",
"first-page": "577",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(21)00160-0_bib3",
"volume": "172",
"year": "2020"
},
{
"DOI": "10.1016/S2589-7500(20)30086-8",
"article-title": "A real-time dashboard of clinical trials for COVID-19",
"author": "Thorlund",
"doi-asserted-by": "crossref",
"first-page": "e286",
"journal-title": "Lancet Digit Health",
"key": "10.1016/S2213-2600(21)00160-0_bib4",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00160-0_bib5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1111/all.14532",
"article-title": "COVID-19 in Severe Asthma Network in Italy (SANI) patients: Clinical features, impact of comorbidities and treatments",
"author": "Heffler",
"doi-asserted-by": "crossref",
"first-page": "887",
"journal-title": "Allergy",
"key": "10.1016/S2213-2600(21)00160-0_bib6",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0243027",
"article-title": "Characteristics and outcomes of COVID-19 patients in New York City's public hospital system",
"author": "Kalyanaraman Marcello",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00160-0_bib7",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30314-3",
"article-title": "Inhaled corticosteroids in virus pandemics: a treatment for COVID-19?",
"author": "Nicolau",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00160-0_bib8",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1183/09031936.00186510",
"article-title": "Effect of different asthma treatments on risk of cold-related exacerbations",
"author": "Reddel",
"doi-asserted-by": "crossref",
"first-page": "584",
"journal-title": "Eur Respir J",
"key": "10.1016/S2213-2600(21)00160-0_bib9",
"volume": "38",
"year": "2011"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "e01648",
"journal-title": "J Virol",
"key": "10.1016/S2213-2600(21)00160-0_bib10",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"article-title": "COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/S2213-2600(21)00160-0_bib11",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0001802",
"article-title": "Validity of the common cold questionnaire (CCQ) in asthma exacerbations",
"author": "Powell",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00160-0_bib12",
"volume": "3",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0194180",
"article-title": "Performance of the inFLUenza Patient-Reported Outcome (FLU-PRO) diary in patients with influenza-like illness (ILI)",
"author": "Powers",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00160-0_bib13",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00160-0_bib14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for COVID-19—Interim WHO solidarity trial results",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00160-0_bib15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkaa501",
"article-title": "Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial",
"author": "Roozbeh",
"doi-asserted-by": "crossref",
"first-page": "753",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/S2213-2600(21)00160-0_bib16",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(20)30383-1",
"article-title": "Projected health-care resource needs for an effective response to COVID-19 in 73 low-income and middle-income countries: a modelling study",
"author": "Tan-Torres Edejer",
"doi-asserted-by": "crossref",
"first-page": "e1372",
"journal-title": "Lancet Glob Health",
"key": "10.1016/S2213-2600(21)00160-0_bib18",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19—preliminary report",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00160-0_bib19",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0116949",
"article-title": "Intensive care unit capacity in low-income countries: a systematic review",
"author": "Murthy",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00160-0_bib20",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1038/d41586-021-00031-0",
"article-title": "Could new COVID variants undermine vaccines? Labs scramble to find out",
"author": "Callaway",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(21)00160-0_bib21",
"volume": "589",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.09.017",
"article-title": "Asthma-associated risk for COVID-19 development",
"author": "Skevaki",
"doi-asserted-by": "crossref",
"first-page": "1295",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/S2213-2600(21)00160-0_bib22",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1136/thx.2010.135210",
"article-title": "Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May–September 2009)",
"author": "Nguyen-Van-Tam",
"doi-asserted-by": "crossref",
"first-page": "645",
"journal-title": "Thorax",
"key": "10.1016/S2213-2600(21)00160-0_bib23",
"volume": "65",
"year": "2010"
},
{
"DOI": "10.1086/503809",
"article-title": "The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking",
"author": "Venarske",
"doi-asserted-by": "crossref",
"first-page": "1536",
"journal-title": "J Infect Dis",
"key": "10.1016/S2213-2600(21)00160-0_bib24",
"volume": "193",
"year": "2006"
},
{
"DOI": "10.1016/j.antiviral.2018.01.012",
"article-title": "Antiviral and anti-inflammatory activity of budesonide against human rhinovirus infection mediated via autophagy activation",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "87",
"journal-title": "Antiviral Res",
"key": "10.1016/S2213-2600(21)00160-0_bib25",
"volume": "151",
"year": "2018"
},
{
"DOI": "10.3390/jcm9113406",
"article-title": "Inhaled corticosteroids and COVID-19 risk and mortality: a nationwide cohort study",
"author": "Choi",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "10.1016/S2213-2600(21)00160-0_bib26",
"volume": "9",
"year": "2020"
},
{
"article-title": "The relationship between asthma, eosinophilia, and outcomes in COVID-19 infection",
"author": "Ho",
"journal-title": "Ann Allergy Asthma Immunol",
"key": "10.1016/S2213-2600(21)00160-0_bib27",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00013-8",
"article-title": "Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK",
"author": "Bloom",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00160-0_bib28",
"year": "2021"
},
{
"DOI": "10.12688/wellcomeopenres.16307.1",
"article-title": "Why the patient-made term ‘long COVID' is needed",
"author": "Perego",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Wellcome Open Res",
"key": "10.1016/S2213-2600(21)00160-0_bib29",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00160-0_bib31",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.jcv.2020.104438",
"article-title": "Validation of SARS-CoV-2 detection across multiple specimen types",
"author": "Perchetti",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Virol",
"key": "10.1016/S2213-2600(21)00160-0_bib32",
"volume": "128",
"year": "2020"
},
{
"DOI": "10.1186/s13063-016-1367-4",
"article-title": "Stopping guidelines for an effectiveness trial: what should the protocol specify?",
"author": "Tyson",
"doi-asserted-by": "crossref",
"first-page": "240",
"journal-title": "Trials",
"key": "10.1016/S2213-2600(21)00160-0_bib33",
"volume": "17",
"year": "2016"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.02.04.21251134",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021001600"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}
