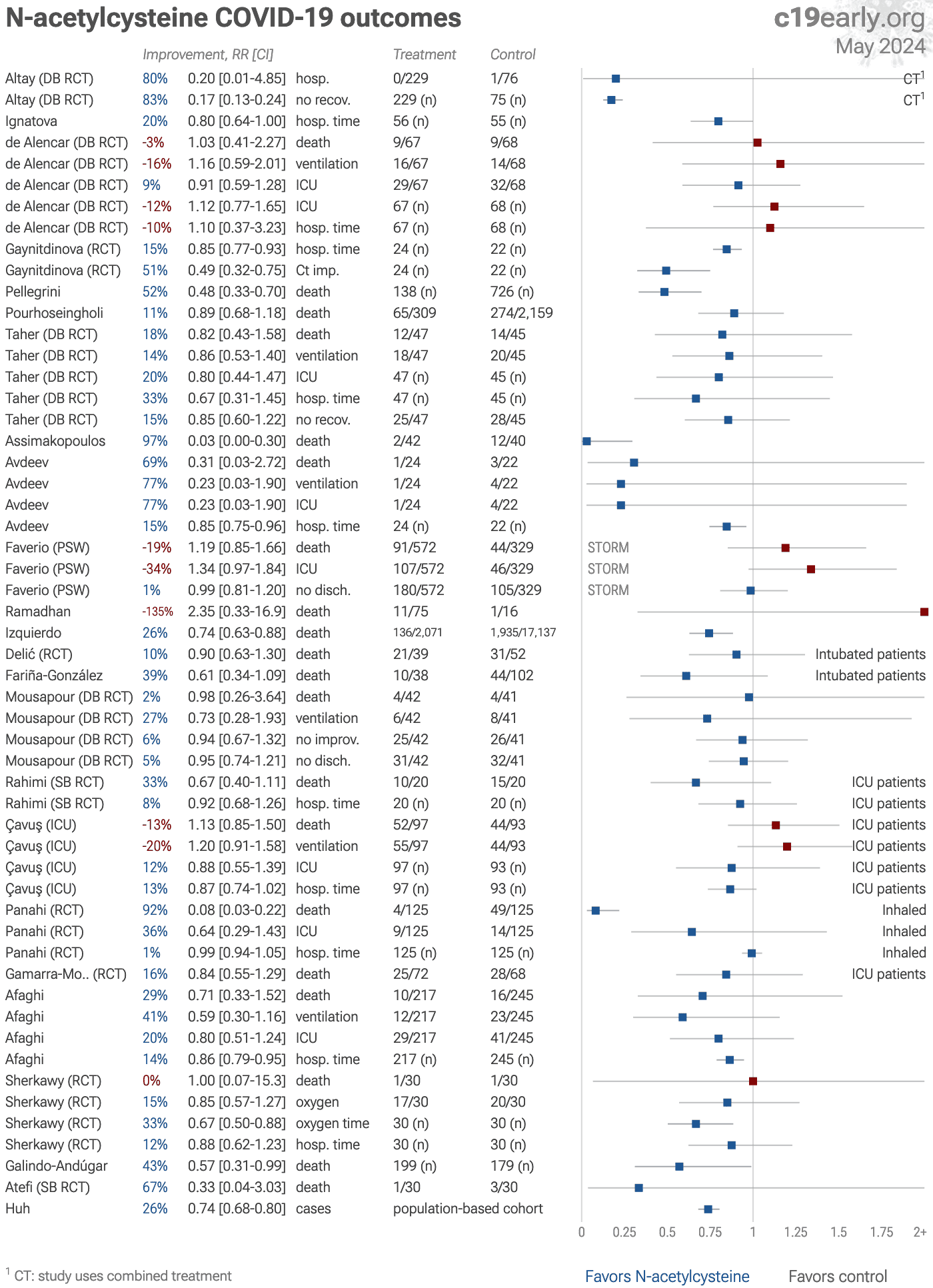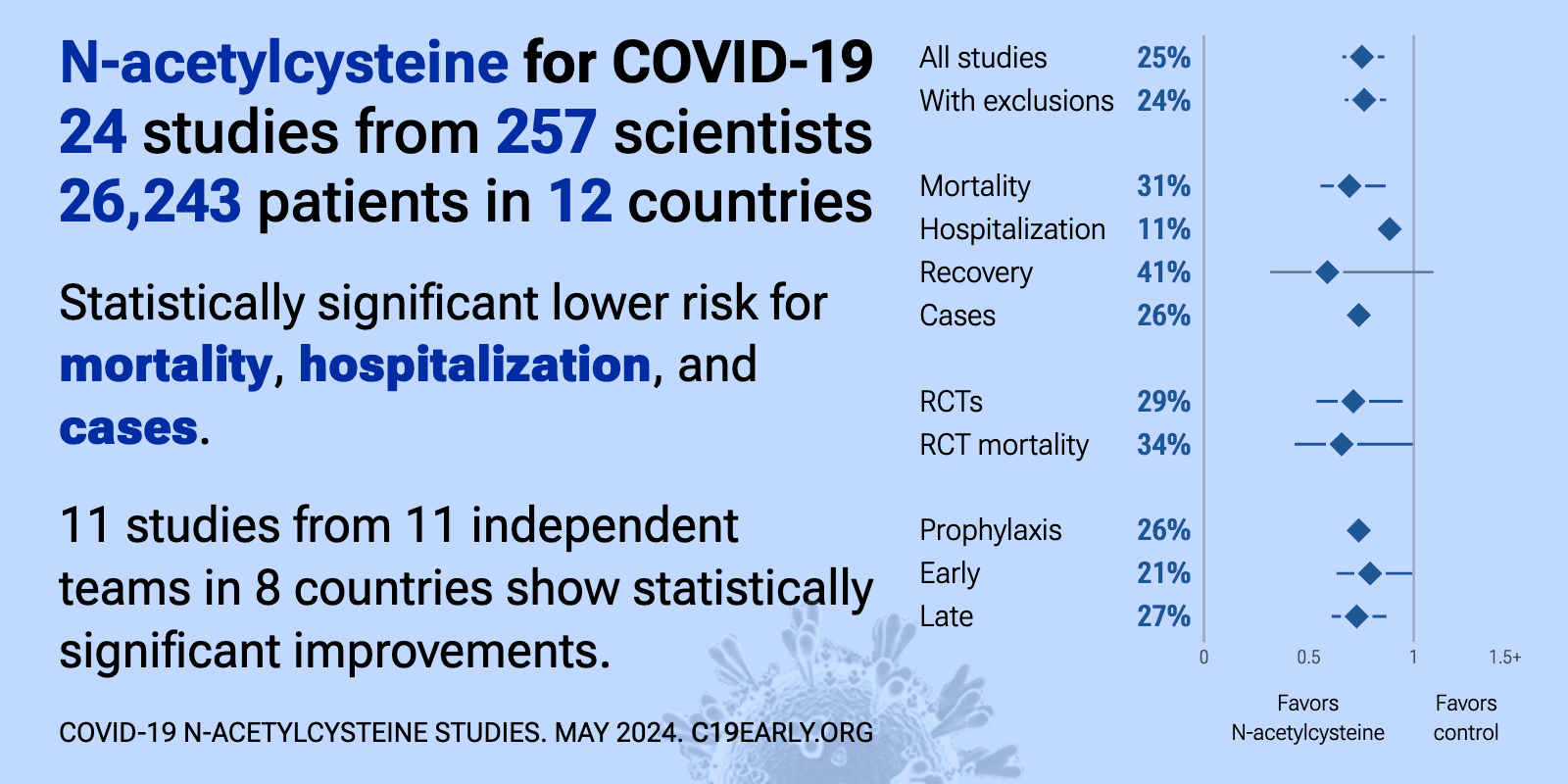
Feb 21 |
N-acetylcysteine reduces COVID-19 risk: real-time meta-analysis of 25 studies (Version 3) | |
| Significantly lower risk is seen for mortality, hospitalization, recovery, and cases. 12 studies from 12 independent teams in 9 countries show significant benefit. Meta-analysis using the most serious outcome reported shows 23% [14&#.. | ||
Dec 2 2025 |
et al., Tạp chí Y học Cộng đồng, doi:10.52163/yhc.v66iCD22.3875 | Study on the effectiveness of molnupiravir regimen combined with n-acetyl cysteine and recourse of respiratory function in patients with mild-moderate COVID-19 |
| 19% improved recovery (p<0.0001). Retrospective 140 mild to moderate COVID-19 outpatients showing shorter treatment duration and improved symptom resolution with the addition of N-acetylcysteine and respiratory rehabilitation versus molnupiravir alone. The combination gro.. | ||
Jul 29 2025 |
et al., Inflammopharmacology, doi:10.1007/s10787-025-01876-x | Effect of N-Acetylcysteine on mortality in COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials |
| 51% lower mortality (p=0.03). Meta analysis of 10 RCTs with 1,424 patients, showing significantly lower mortality with N-acetylcysteine. | ||
Jul 22 2025 |
et al., BMC Pulmonary Medicine, doi:10.1186/s12890-025-03824-5 | Efficacy and safety of combined nebulization of unfractionated heparin, acetylcysteine, budesonide and ipratropium bromide in hospitalised patients with COVID-19 pneumonia: a randomized controlled clinical trial |
| RCT 74 hospitalized COVID-19 pneumonia patients showing improved lung lesion absorption and oxygenation with combined nebulization of unfractionated heparin, acetylcysteine, budesonide, and ipratropium bromide. The treatment group demonst.. | ||
Apr 7 2025 |
et al., Scientific Reports, doi:10.1038/s41598-025-92242-y | Antiviral effect of Bromelain combined with acetylcysteine against SARS-CoV-2 Omicron variant |
| In vitro and ex vivo study showing that BromAc (bromelain and N-acetylcysteine) exhibits antiviral activity against SARS-CoV-2 Omicron variant. Authors demonstrate that BromAc at 250 μg/mL significantly reduces infectious viral particles .. | ||
Jan 16 2025 |
et al., Life, doi:10.3390/life15010113 | Immune-Boosting and Antiviral Effects of Antioxidants in COVID-19 Pneumonia: A Therapeutic Perspective |
| Review of immune-boosting and antiviral effects of antioxidants in COVID-19 pneumonia. Authors provide an overview of the literature on the use of antioxidants, including vitamins, trace elements, ozone, glutathione, L-carnitine, melatoni.. | ||
Nov 8 2024 |
et al., Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6 | The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death |
| Review of the effects of reactive oxygen species (ROS) on cell death pathways in SARS-CoV-2 infection. SARS-CoV-2 induces oxidative stress and ROS generation which can lead to several types of regulated cell death including NETosis, ferro.. | ||
Aug 11 2024 |
et al., Cells, doi:10.3390/cells13161331 | Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling |
| In vitro study showing that the SARS-CoV-2 spike protein can activate cardiac fibroblasts through ACE2-dependent mechanisms, leading to cardiac fibrosis via the NLRP3 inflammasome and NF-κB signaling pathways. The results suggest that COV.. | ||
Jul 1 2024 |
et al., Phytomedicine, doi:10.1016/j.phymed.2024.156279 (date from preprint) | Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC) |
| In vitro study showing andrographolide attenuates infection of SARS-CoV-2 wildtype and omicron variants in human lung epithelial cells and monkey kidney cells. Proteomic analysis revealed andrographolide induces expression of the glutathi.. | ||
Jun 1 2024 |
et al., QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcae070.076 | Safety and Efficacy of N-Acetylcysteine as an Adjuvant Therapy in Critically Ill COVID-19 Patients |
| Retrospective 80 ICU patients reporting no statistically significant differences with NAC treatment, however the actual results are not provided. The study is described as a retrospective observational study however authors also state tha.. | ||
Apr 16 2024 |
et al., ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1 | Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases |
| In silico study identifying potential drugs beneficial for COVID-19 by integrating transcriptomics, proteomics, metabolomics, lipidomics, and drug data. Authors explore interactions between drugs, molecular features, and disease severity... | ||
Apr 6 2024 |
et al., Journal of Comprehensive Pediatrics, doi:10.5812/jcp-139612 | Efficacy of N‑Acetylcysteine in Children with Moderate COVID-19: A Placebo-Controlled Randomized Clinical Trial |
| 44% shorter hospitalization (p=0.008). RCT 58 hospitalized children with moderate COVID-19 showing shorter hospitalization and improved oxygen saturation with N-acetylcysteine treatment. However, baseline oxygen values are inconsistent with the reported inclusion criteria - th.. | ||
Feb 1 2024 |
et al., Journal of Molecular Cell Biology. doi:10.1093/jmcb/mjae004 | Identification of druggable host dependency factors shared by multiple SARS-CoV-2 variants of concern |
| In vitro study showing that inhibition of host cell factors rather than viral elements may prevent emergence of SARS-CoV-2 variants resistant to current drugs. Authors identified host genes/proteins commonly required for infection by majo.. | ||
Dec 13 2023 |
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1308477 | Taming the SARS-CoV-2-mediated proinflammatory response with BromAc® |
| In vitro study of peripheral blood cells from 9 healthy donors showing reduced proinflammatory cytokines, growth factors, and regulatory cytokines; decreased neutrophils and monocytes; and increased HLA-DR expression on monocytes with Bro.. | ||
Nov 20 2023 |
et al., Immunity, Inflammation and Disease, doi:10.1002/iid3.1083 | Evaluation of the efficacy and safety of oral N‐acetylcysteine in patients with COVID‐19 receiving the routine antiviral and hydroxychloroquine protocol: A randomized controlled clinical trial |
| 67% lower mortality (p=0.61). RCT 60 hospitalized COVID-19 patients evaluating the efficacy and safety of adding oral N-acetylcysteine (NAC) at 600mg three times daily to standard antiviral treatment regimens. The NAC group showed significantly greater reduction in C-.. | ||
Oct 4 2023 |
et al., International Journal of Medical Laboratory, doi:10.18502/ijml.v10i3.13746 | The Effects of Vitamin D3 and N-Acetylcysteine Administration in Patients with COVID-19 Hospitalization in the Iranian Population |
| RCT 100 hospitalized patients reporting shorter hospitalization with vitamin D + N-acetylcysteine. However, day 28 SaO2 is not consistent with the reported recovery values. For three arms the day 28 values are worse than the baseline valu.. | ||
Sep 20 2023 |
et al., Signal Transduction and Targeted Therapy, doi:10.1038/s41392-023-01580-8 | The role of cell death in SARS-CoV-2 infection |
| Review of cell death pathways in SARS-CoV-2 infection. Authors note that N-acetylcysteine (NAC) is a precursor to glutathione that may inhibit ferroptosis. Ferroptosis is a regulated cell death triggered by oxidative stress that has been .. | ||
Jul 21 2023 |
et al., Revista Clínica Española, doi:10.1016/j.rceng.2023.07.006 | Impact of N-Acetylcysteine in the mortality of patients hospitalized with COVID-19: a retrospective cohort study |
| 43% lower mortality (p=0.05). Retrospective 378 hospitalized patients in Spain, showing lower mortality with N-acetylcysteine treatment. | ||
Jun 30 2023 |
et al., Journal of Advanced Veterinary and Animal Research, doi:10.5455/javar.2023.j665 | N-acetylcysteine reduces severity and mortality in COVID-19 patients: A systematic review and meta-analysis |
| 35% lower mortality (p<0.0001). Systematic review and meta analysis showing lower mortality with N-acetylcysteine treatment. | ||
Jun 1 2023 |
et al., Archives of Pharmaceutical Sciences Ain Shams University, doi:10.21608/aps.2023.212265.1122 | Impact of N-Acetylcysteine on Modulating Inflammation in Patients Hospitalized with Moderate COVID-19 Infections: A Prospective Randomized Trial |
| 15% lower need for oxygen therapy (p=0.6) and 12% shorter hospitalization (p=0.45). RCT 60 hospitalized patients showing that oral N-acetylcysteine (NAC) at 1800mg daily significantly decreased plasma TNF-α levels and increased glutathione peroxidase levels. The NAC group had a shorter duration of oxygen support, while t.. | ||
Jun 1 2023 |
et al., Caspian J Intern Med, doi:10.22088/cjim.14.3.553 | N-acetylcysteine as adjuvant therapy for hospitalized Covid-19 patients: A single-center prospective cohort study |
| 29% lower mortality (p=0.42), 41% lower ventilation (p=0.16), 20% lower ICU admission (p=0.36), and 14% shorter hospitalization (p=0.002). Prospective study of 217 patients treated with NAC and 245 matched controls, showing improved recovery with treatment. 1500mg intravenous NAC daily. | ||
May 19 2023 |
et al., Narra J, doi:10.52225/narra.v3i2.121 | The role of N-acetylcysteine in decreasing neutrophil-lymphocyte ratio in COVID-19 patients: A double-blind, randomized controlled trial |
| RCT 40 moderate/severe COVID-19 patients in Indonesia, showing significantly lower NLR with N-acetylcysteine treatment. | ||
May 8 2023 |
et al., Nutrients, doi:10.3390/nu15092235 | Response to Intravenous N-Acetylcysteine Supplementation in Critically Ill Patients with COVID-19 |
| 16% lower mortality (p=0.49). RCT 140 ICU patients in Spain, 72 treated with N-acetylcysteine (NAC). NAC patients showed improved PaO2/FiO2, CRP, D-dimer, and LDH, and there were associations between glutathione and clinical outcomes and severity biomarkers in NAC-tre.. | ||
Apr 14 2023 |
et al., Pharmaceuticals, doi:10.3390/ph16040591 | N-Acetyl Cysteine Restores the Diminished Activity of the Antioxidant Enzymatic System Caused by SARS-CoV-2 Infection: Preliminary Findings |
| Prospective analysis of 16 COVID-19 patients treated with NAC and 20 healthy controls, showing that NAC may help restore diminished activity of the antioxidant enzymatic system in patients with COVID-19. | ||
Mar 15 2023 |
et al., Inflammopharmacology, doi:10.1007/s10787-023-01183-3 | Nutritional deficiencies that may predispose to long COVID |
| Review of 22 nutritional factors that have been linked to COVID-19 outcomes, the role of nutrients in COVID-19 infection, and the prevalence of multiple nutritional deficiencies in the population. | ||
Feb 13 2023 |
et al., Advances in Animal and Veterinary Sciences, doi:10.17582/journal.aavs/2023/11.3.404.409 | The Potential of Melatonin and N-Acetylcysteine on Remdesivir Induced Liver Injury in Covid 19 Patients |
| RCT 70 hospitalized COVID-19 patients evaluating the effects of N-acetylcysteine (NAC) and melatonin on liver injury induced by remdesivir treatment. NAC 600mg twice daily and melatonin 6mg daily. All patients received remdesivir. Liver e.. | ||
Jan 3 2023 |
et al., Research Square, doi:10.21203/rs.3.rs-2309373/v2 | Evaluation of the recovery rate and prevention of hospitalization among covid-19 outpatients: a randomized clinical trial comparing N-acetylcysteine with Bromhexine |
| 93% lower mortality (p=0.01), 78% lower hospitalization (p<0.0001), and 16% faster recovery (p=0.02). RCT 225 outpatients in Iran showing lower mortality and hospitalization, and faster recovery with N-acetylcysteine and bromhexine. Baseline information per group is not provided, Figure 1 has the control group hospitalization status switc.. | ||
Dec 19 2022 |
et al., Journal of Medical Virology, doi:10.1002/jmv.28393 | Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: An open‐label randomized controlled clinical trial |
| 92% lower mortality (p<0.0001), 36% lower ICU admission (p=0.38), and 1% shorter hospitalization (p=0.81). RCT 250 hospitalized COVID-19 patients showing reduced mortality rate and inflammatory markers with N-acetylcysteine (NAC) 400μg inhaled spray twice daily for 7 days as adjunctive treatment. There was no significant difference in hospital.. | ||
Oct 25 2022 |
et al., Aksaray Üniversitesi Tıp Bilimleri Dergisi, 3:2 | Kritik COVID-19 hastalarında kullanılan N-asetilsisteinin(NAC) klinik bulgulara, inflamatuar parametrelere böbrek fonksiyonlarına olan etkileri |
| 13% higher mortality (p=0.47), 20% higher ventilation (p=0.25), 12% shorter ICU admission (p=0.58), and 13% shorter hospitalization (p=0.09). Retrospective 190 critical COVID-19 patients in Turkey, showing no significant differences with N-acetylcysteine treatment in unadjusted results with no baseline details. NAC 2400mg/day. | ||
Oct 21 2022 |
et al., Cells, doi:10.3390/cells11203313 | Inhibition of the Cell Uptake of Delta and Omicron SARS-CoV-2 Pseudoviruses by N-Acetylcysteine Irrespective of the Oxidoreductive Environment |
| ACE2-HEK293 in vitro study showing dose-dependent inhibition of the uptake of delta and omicron SARS-CoV-2 pseudoviruses with N-acetylcysteine. | ||
Oct 8 2022 |
et al., Jundishapur Journal of Natural Pharmaceutical Products, doi:10.5812/jjnpp-129817 | Efficacy of N-acetyl Cysteine in Severe COVID-19 Patients: A Randomized Controlled Phase III Clinical Trial |
| 33% lower mortality (p=0.19) and 8% shorter hospitalization (p=0.63). RCT 40 ICU patients in Iran, showing lower mortality with NAC treatment, without statistical significance. Single dose intravenous NAC 300 mg/kg. | ||
Jun 20 2022 |
et al., Gastroenterology and Hepatology from Bed to Bench, doi:10.22037/ghfbb.v15i3.2565 | Efficacy and Safety of Acetylcysteine for the Prevention of Liver Injury in Covid-19 Intensive Care Unit Patients Under Treatment with Remdesivir: A Double-Blind, Placebo-Controlled Randomized Clinical Trial: Prevention of liver injury in severe Covid-19 pneumonia |
| 2% lower mortality (p=1), 27% lower ventilation (p=0.57), 6% greater improvement (p=0.82), and 5% higher hospital discharge (p=0.8). RCT 83 severe COVID-19 pnuemonia patients in Iran, 42 treated with acetylcysteine, showing no significant difference in clinical outcomes. All patients received remdesivir, famotidine, and vitamin C. More patients were at baseline categor.. | ||
May 31 2022 |
et al., Journal of Intensive Care Medicine, doi:10.1177/08850666221105423 | Hourly Analysis of Mechanical Ventilation Parameters in Critically Ill Adult Covid-19 Patients: Association with Mortality |
| 39% lower mortality (p=0.08). Retrospective 140 mechanically ventilated patients in Spain, showing lower mortality with acetylcysteine treatment in unadjusted results, not reaching statistical significance. | ||
May 28 2022 |
et al., Microorganisms, doi:10.3390/microorganisms10061118 | Effects of Different Inhalation Therapy on Ventilator-Associated Pneumonia in Ventilated COVID-19 Patients: A Randomized Controlled Trial |
| 14% lower mortality (p=0.37). RCT mechanically ventilated patients in Croatia, 39 treated with N-acetylcysteine and 52 control patients, showing no significant difference in mortality with treatment. Treated patients showed a lower incidence of gram-positive or MRSA-c.. | ||
Jan 27 2022 |
et al., Science Progress, doi:10.1177/00368504221074574 | Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19 |
| 26% lower mortality (p=0.0007). Retrospective 19,208 COVID+ hospitalized patients in Spain, 2,071 treated with high dose NAC, showing lower mortality with treatment. In multivariable analysis, authors adjust for corticosteroids, but do not adjust for HCQ use which was a.. | ||
Jan 21 2022 |
et al., European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022 | Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants |
| In vitro study testing combinations of plant extracts and micronutrients with several variants of SARS-CoV-2. A combination of vitamin C, N-acetylcysteine, curcumin, quercetin, resveratrol, theaflavin, naringenin, baicalin, and broccoli e.. | ||
Dec 27 2021 |
et al., Indonesian Journal of Tropical and Infectious Disease, 9:3 | The Effects of N-Acetylcysteine as Adjuvant Therapy To Reduce TNF-Α Level And Increase SPO2/FIO2 Ratio In Improving Hypoxemia In COVID-19 Patients |
| 135% higher mortality (p=0.68). Prospective study with 75 NAC patients and 16 control patients, showing no significant difference in mortality. | ||
Dec 2 2021 |
et al., ERJ Open Research, doi:10.1183/23120541.00542-2021 | Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study |
| 19% higher mortality (p=0.33), 34% higher ICU admission (p=0.08), and 1% higher hospital discharge (p=0.94). Retrospective 1,083 consecutive hospitalized COVID patients in Italy, showing no significant differences with NAC treatment. The number of patients transferred to another facility exceeds the number of deaths, which may significantly affe.. | ||
Nov 10 2021 |
et al., Russian Medical Inquiry, doi:10.32364/2587-6821-2021-5-7-473-478 | Therapeutic possibilities of using an expectorant mucolytic agent with antioxidant properties in COVID-19 infection |
| 20% shorter hospitalization (p=0.05). Retrospective 111 patients with moderate COVID-19 pneumonia, 56 treated with NAC, showing shorter hospitalization time with treatment. NAC 1200mg daily intravenous, divided into two doses. | ||
Jul 9 2021 |
et al., Journal of Infection, doi:10.1016/j.jinf.2021.07.003 | N-acetylcysteine for the treatment of COVID-19 among hospitalized patients |
| 69% lower mortality (p=0.34), 77% lower ventilation (p=0.18), 77% lower ICU admission (p=0.18), and 15% shorter hospitalization (p=0.01). Prospective study of 24 hospitalized COVID-19 patients in Russia treated with NAC, and 22 matched controls, showing significantly improved SpO2/FiO2, and significantly shorter hospitalization with treatment. | ||
Jun 29 2021 |
et al., Infectious Diseases, doi:10.1080/23744235.2021.1945675 | N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study |
| 97% lower mortality (p=0.006). Retrospective 42 hospitalized PCR+ COVID-19 pneumonia patients treated with NAC, and a matched control group of 40 patients, showing significantly lower severe respiratory failure and significantly lower mortality with treatment. NAC 600 .. | ||
Jun 28 2021 |
et al., Advanced Science, doi:10.1002/advs.202101222 | Combined Metabolic Activators Accelerates Recovery in Mild-to-Moderate COVID-19 |
| 80% lower hospitalization (p=0.25) and 83% improved recovery (p<0.0001). RCT 304 low-risk outpatients, 229 treated with N-acetylcysteine, l-carnitine tartrate, nicotinamide riboside chloride, and serine, showing significantly faster recovery with treatment. Plasma levels of proteins and metabolites associated .. | ||
Jun 10 2021 |
et al., Pharmacological Reports, doi:10.1007/s43440-021-00296-2 | A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome |
| 18% lower mortality (p=0.65), 14% lower ventilation (p=0.67), 20% shorter ICU admission (p=0.48), and 33% shorter hospitalization (p=0.31). RCT 92 hospitalized patients, 47 treated with NAC, showing non-significant improvements in outcomes. IRCT20120215009014N355. NAC 40mg/kg/day intravenous for 3 days. | ||
May 26 2021 |
et al., Research Square, doi:10.21203/rs.3.rs-365321/v2 | Case Characteristics, Clinical Data, And Outcomes of Hospitalized COVID-19 Patients In Qom Province, Iran: A Prospective Cohort Study |
| 11% lower mortality (p=0.43). Prospective study of 2,468 hospitalized COVID-19 patients in Iran, showing no significant difference with NAC treatment. IR.MUQ.REC.1399.013. | ||
May 23 2021 |
et al., Gastroenterology, doi:10.1016/S0016-5085(21)02756-6 | A Retrospective Analysis of Outcomes Amongst COVID-19 Infected Patients with Acute Hepatitis Receiving N-Acetylcysteine Therapy in a Safety Net Hospital |
| 52% lower mortality (p=0.0001). Retrospective 864 hospitalized late stage COVID-19 patients in the USA, 138 receiving NAC treatment for acute hepatitis, showing lower mortality with treatment. Results are adjusted for confounders, however details are not provided. | ||
Apr 20 2021 |
et al., Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2021.1910570 | The in silico mechanism of hVKOR interaction with acetaminophen and its metabolite, as well as N-acetyl cysteine: caution on application in COVID-19 patients |
| In silico study suggesting increased risk of cerebral hemorrhage with acetaminophen use for COVID-19. Authors show acetaminophen metabolite N-acetyl-p-benzoquinone imine (NAPQI), and to a lesser extent N-acetylcysteine (NAC), bind to hum.. | ||
Mar 6 2021 |
et al., Viruses, doi:10.3390/v13030425 | The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2 |
| In vitro study showing dose dependent inactivation of SARS-CoV-2 with the combination of bromelain and acetylcysteine. | ||
Feb 19 2021 |
et al., Pulmonologiya, doi:10.18093/0869-0189-2021-31-1-21-29 | N-acetylcysteine as a part of complex treatment of moderate COVID-associated pneumonia |
| 15% shorter hospitalization (p=0.001) and 51% improved recovery (p=0.001). RCT 46 hospitalized patients with moderate COVID-19 pneumonia, 24 treated with N-acetylcysteine, showing significantly shorter hospitalization with treatment. NAC 1,200 - 1,500mg/day intravenously. | ||
Nov 30 2020 |
et al., Therapeutics and Clinical Risk Management, doi:10.2147/TCRM.S273700 | N-Acetylcysteine to Combat COVID-19: An Evidence Review |
| Review of N-acetylcysteine (NAC) as a potential treatment for COVID-19. Authors propose that NAC's antioxidant, anti-inflammatory, and immune-modulating properties could benefit COVID-19 patients by suppressing neutrophil activation, redu.. | ||
Sep 23 2020 |
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciaa1443 | Double-blind, Randomized, Placebo-controlled Trial With N-acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19) |
| 3% higher mortality (p=0.94), 16% higher ventilation (p=0.64), 9% lower ICU admission (p=0.65), and 10% longer hospitalization (p=0.87). RCT 135 severe stage patients in Brazil, showing no significant differences. NAC 21g (~300mg/kg) for 20 hours. U1111-1250-356 [ensaiosclinicos.gov.br]. | ||
May 4 2020 |
et al., medRxiv, doi:10.1101/2020.05.04.20089904 | Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea |
| 28% fewer cases (p<0.0001). Retrospective database analysis of 65,149 in South Korea, showing significantly lower cases with existing N-acetylcysteine treatment. The journal version of this paper does not present the N-acetylcysteine results. | ||
Aug 15 2017 |
et al., Circulation, doi:10.1161/CIRCULATIONAHA.117.027290 | Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi |
| Experimental study in mice showing potent thrombolytic effects with N-acetylcysteine (NAC) in arterial thrombosis models. Authors hypothesize that NAC's thrombolytic mechanism involves destabilizing the VWF-dependent outer portion of thro.. | ||
Feb 1 2011 |
et al., Journal of Clinical Investigation, doi:10.1172/JCI41062 | N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice |
| Experimental study using purified human vWF, human plasma, and wild-type/ADAMTS13-deficient mice showing that N-acetylcysteine (NAC) reduces von Willebrand factor multimer size and activity. | ||
Jul 1 1997 |
et al., European Respiratory Journal, doi:10.1183/09031936.97.10071535 | Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment |
| RCT 262 elderly and/or chronic disease patients in Italy showing significantly reduced frequency and severity of influenza-like episodes with 600mg N-acetylcysteine twice daily for 6 months. Seroconversion to influenza A/H1N1 was similar .. | ||