Study on the effectiveness of molnupiravir regimen combined with n-acetyl cysteine and recourse of respiratory function in patients with mild-moderate COVID-19
et al., Tạp chí Y học Cộng đồng, doi:10.52163/yhc.v66iCD22.3875, Dec 2025
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
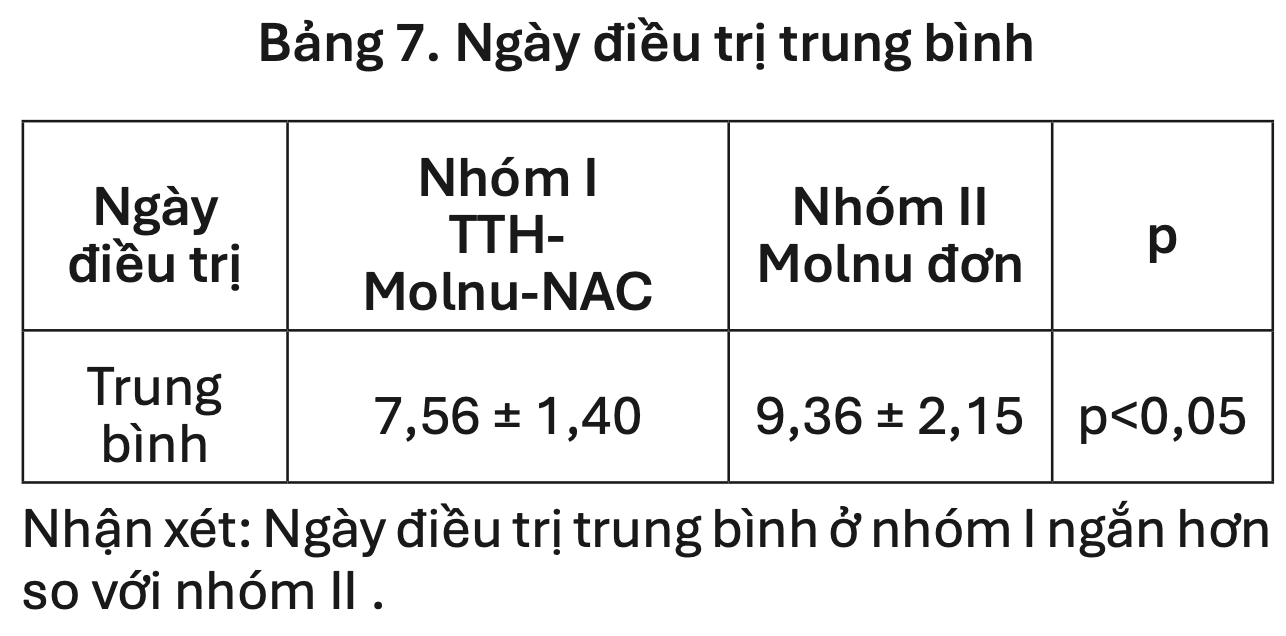
Retrospective 140 mild to moderate COVID-19 outpatients showing shorter treatment duration and improved symptom resolution with the addition of N-acetylcysteine and respiratory rehabilitation versus molnupiravir alone. The combination group (n=80) had mean treatment duration of 7.56±1.40 days versus 9.36±2.15 days for molnupiravir monotherapy (n=60, p<0.05).
|
risk of no recovery, 19.2% lower, RR 0.81, p < 0.001, treatment mean 7.56 (±1.4) n=80, control mean 9.36 (±2.15) n=60.
|
|
risk of no viral clearance, 62.5% lower, RR 0.38, p = 0.58, treatment 1 of 80 (1.2%), control 2 of 60 (3.3%), NNT 48.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Nghĩa et al., 2 Dec 2025, retrospective, Vietnam, peer-reviewed, 3 authors.
Contact: bsminhquan@gmail.com.
42. NGHIÊN CỨU HIỆU QUẢ CỦA PHÁC ĐỒ MOLNUPIRAVIR KẾT HỢP N-ACETYL CYSTEINE VÀ PHỤC HỒI CHỨC NĂNG HÔ HẤP Ở BỆNH NHÂN COVID-19 MỨC ĐỘ TRUNG BÌNH NHẸ
Tạp chí Y học Cộng đồng, doi:10.52163/yhc.v66icd22.3875
COVID-19 is a disease caused by the novel coronavirus SARS-CoV-2. It can cause a range of symptoms, including fever, cough, loss of smell and taste, and more. Some people have mild illness with few symptoms, while others have more severe illness. Typical symptoms include fever, cough, and fatigue. Other symptoms may include: shortness of breath, loss of smell or taste, body aches, headache, sore throat, runny or stuffy nose, and gastrointestinal symptoms such as nausea, vomiting, or diarrhea.
Object: (1). Identification of clinical symptoms in Covid-19 patients. (2). Evaluation of changes in clinical symptoms and viral levels in COVID-19 patients treated with molnupiravir plus NAC and diaphragmatic breathing techniques comparing molnupiravir monotherapy. Method study: We selected 140 mild and moderate COVID-19 patients. Criteria for diagnosis and classification of COVID-19 severity according to the Ministry of Health in 2021 and divided into 2 groups: group I consists of 80 patients, who have received molnupiravir combined N-acetyl cysteine therapy and diaphragmatic breathing techniques, group II consists of 60 patients, received molnupiravir alone. Descriptive research methods. Results: Common age 18-<50 years old 80.57%, more female than male. The clinical symptoms of Covid-19 are very common, chest pain, mild shortness of breath, fever, loss of taste and smell, and fatigue. Significant changes in clinical symptoms and viral levels after treatment in 2 patients groups. Symptoms of fatigue, headache, loss of taste and smell changed little in the molnupiravir monotherapy group. Conclusion: Molnupiravir regimen combined with N-Acetyl Cysteine and Rehabilitation of respiratory function clearly changed clinical symptoms and viral concentrations, shortening the duration of treatment.
DOI record:
{
"DOI": "10.52163/yhc.v66icd22.3875",
"ISSN": [
"2354-0613"
],
"URL": "http://dx.doi.org/10.52163/yhc.v66iCD22.3875",
"abstract": "<jats:p>Muc tiêu: 1). Xác định các triệu chứng lâm sàng ở bệnh nhân COVID-19. (2). Đánh giá sự thay đổi các triệu chứng lâm sàng và nồng độ virus ở bệnh nhân COVID-19 được điều trị bằng liệu pháp Molnupiravir kết hợp NAC và kỹ thuật tập thở cơ hoành so sánh liệu pháp Molnupiravir đơn.\r\nPhương pháp nghiên cứu: Chúng tôi chọn 140 bệnh nhân COVID-19 thể nhẹ và trung bình. Tiêu chuẩn chẩn đoán và phân loại mức độ COVID-19 theo Bộ y tế 2021 và được chia làm 2 nhóm: Nhóm I 80 bệnh nhân dùng liệu pháp Molnupiravir kết hợp N-acetyl cysteine và phục hồi chức năng hô hấp; Nhóm II 60 bệnh nhân dùng Molnupiravir đơn thuần. Phương pháp nghiên cứu mô tả.\r\nKết quả: Tuổi thường gặp 18-< 50 tuổi 80,57% nữ gặp nhiều hơn nam các triệu chứng lâm sàng Covid-19 rất thường gặp đau tức ngực, khó thở nhẹ, sốt, mất vị giác và khứu giác, mệt mỏi. Thay đổi đáng kể các triệu chứng lâm sàng và nồng độ virus sau điều trị ở 2 nhóm bệnh nhân. Triệu chứng mệt mỏi, đau đầu, mất vị giác và khứu giác thay đổi ít ở nhóm molnupiravir đơn trị. Ngày điều trị trung bình ở nhóm molnupiravir kết hợp N-acetyl cysteine và phục hồi chức năng hô hấp ngắn hơn so với molnupiravir đơn thuần.\r\nKết luận: Phác đồ Molnupiravir kết hợp N-Acetyl Cysteine và phục hồi chức năng hô hấp làm thay đổi rõ ràng các triệu chứng lâm sàng và nồng đọ virus, rút ngắn thời gian điều trị.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Nghĩa",
"given": "Huỳnh Đình",
"sequence": "first"
},
{
"affiliation": [],
"family": "Phi",
"given": "Trương Dương",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thanh",
"given": "Đỗ Phúc",
"sequence": "additional"
}
],
"container-title": "Tạp chí Y học Cộng đồng",
"container-title-short": "YHCĐ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
12,
3
]
],
"date-time": "2025-12-03T04:19:26Z",
"timestamp": 1764735566000
},
"deposited": {
"date-parts": [
[
2025,
12,
4
]
],
"date-time": "2025-12-04T04:19:32Z",
"timestamp": 1764821972000
},
"indexed": {
"date-parts": [
[
2025,
12,
4
]
],
"date-time": "2025-12-04T06:45:59Z",
"timestamp": 1764830759501,
"version": "3.46.0"
},
"is-referenced-by-count": 0,
"issue": "CĐ22-Hội Hóa sinh Y học Việt Nam",
"issued": {
"date-parts": [
[
2025,
12,
2
]
]
},
"journal-issue": {
"issue": "CĐ22-Hội Hóa sinh Y học Việt Nam",
"published-online": {
"date-parts": [
[
2025,
11,
27
]
]
}
},
"link": [
{
"URL": "https://tapchiyhcd.vn/index.php/yhcd/article/download/3875/3538",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://tapchiyhcd.vn/index.php/yhcd/article/download/3875/3538",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "29820",
"original-title": [],
"prefix": "10.52163",
"published": {
"date-parts": [
[
2025,
12,
2
]
]
},
"published-online": {
"date-parts": [
[
2025,
12,
2
]
]
},
"publisher": "Institute of Community Health",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://tapchiyhcd.vn/index.php/yhcd/article/view/3875"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "42. NGHIÊN CỨU HIỆU QUẢ CỦA PHÁC ĐỒ MOLNUPIRAVIR KẾT HỢP N-ACETYL CYSTEINE VÀ PHỤC HỒI CHỨC NĂNG HÔ HẤP Ở BỆNH NHÂN COVID-19 MỨC ĐỘ TRUNG BÌNH NHẸ",
"type": "journal-article",
"volume": "66"
}
