Efficacy of N-acetyl Cysteine in Severe COVID-19 Patients: A Randomized Controlled Phase III Clinical Trial
et al., Jundishapur Journal of Natural Pharmaceutical Products, doi:10.5812/jjnpp-129817, Oct 2022
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
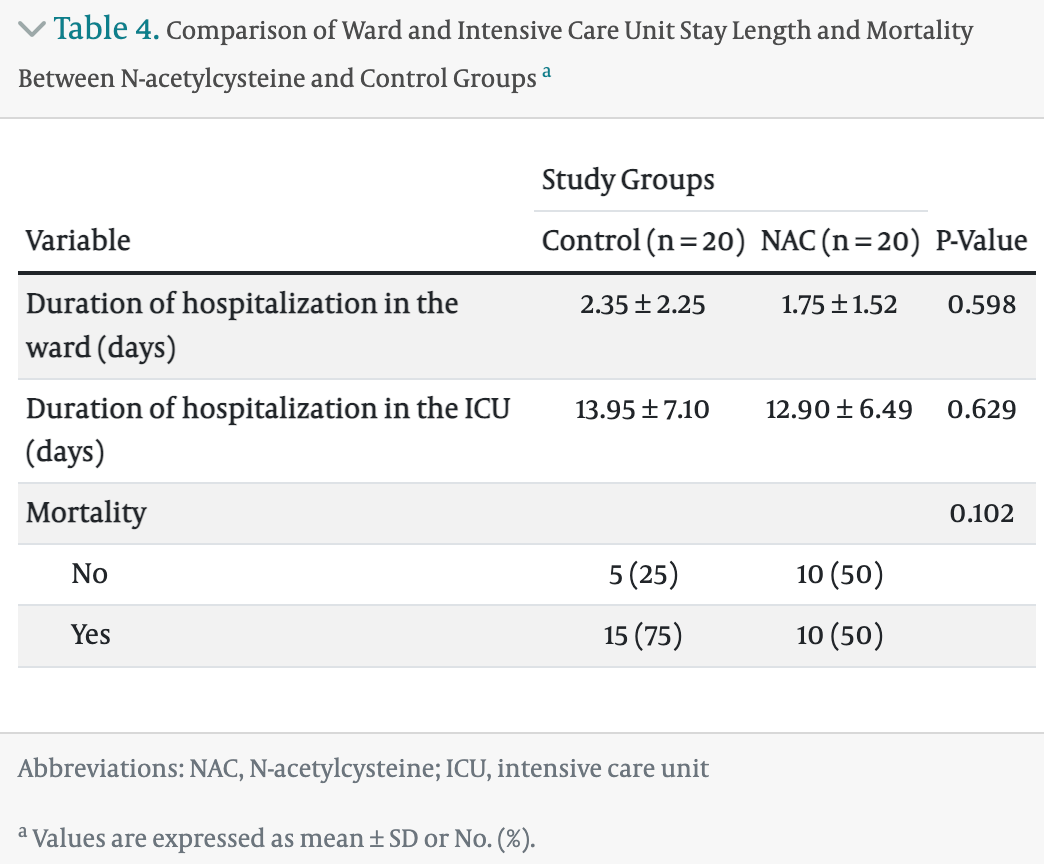
RCT 40 ICU patients in Iran, showing lower mortality with NAC treatment, without statistical significance. Single dose intravenous NAC 300 mg/kg.
Although the 33% lower mortality is not statistically significant, it is consistent with the significant 31% lower mortality [14‑44%] from meta-analysis of the 20 mortality results to date.
|
risk of death, 33.3% lower, RR 0.67, p = 0.19, treatment 10 of 20 (50.0%), control 15 of 20 (75.0%), NNT 4.0.
|
|
hospitalization time, 7.5% lower, relative time 0.92, p = 0.63, treatment 20, control 20.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rahimi et al., 8 Oct 2022, Single Blind Randomized Controlled Trial, Iran, peer-reviewed, 10 authors.
Efficacy of N-acetyl Cysteine in Severe COVID-19 Patients: A Randomized Controlled Phase III Clinical Trial
Jundishapur Journal of Natural Pharmaceutical Products, doi:10.5812/jjnpp-129817
Background: Today, various drugs have been investigated as the primary or complementary treatment for coronavirus disease 2019 . N-acetylcysteine (NAC) has been used as a mucolytic in pulmonary diseases. This drug apparently contributes to the retrieval of the intracellular antioxidant system. Objectives: This study aimed to determine the efficacy of NAC in severe COVID-19 patients admitted to the intensive care unit (ICU). Methods: This single-blinded randomized controlled phase III clinical trial included 40 patients with confirmed COVID-19 (based on polymerase chain reaction) admitted to the Shahid Mohammadi Hospital's ICU, Bandar Abbas, Iran, in 2020. All cases had severe COVID-19. They were allocated randomly to two equal groups. Patients in the control group received standard drug therapy based on the treatment protocol of the national COVID-19 committee, while those in the NAC group received a single dose of intravenous NAC (300 mg/kg) upon admission to the ICU in addition to standard drug treatment. Clinical status and laboratory tests were done on admission to the ICU and then 14 days later or at discharge without knowing the patient grouping. Results: The two groups were comparable regarding age, gender, and other baseline laboratory and clinical parameters. At the final evaluation, respiratory rate (21.25 ± 4.67 vs. 27.37 ± 6.99 /min) and D-dimer (186.37 ± 410.23 vs. 1339.04 ± 2183.87 ng/mL) were significantly lower in the NAC group (P = 0.004 and P = 0.030, respectively). Also, a lower percentage of patients in the NAC group had lactate dehydrogenase (LDH) ≤ 245 U/L (0% vs. 25%, P = 0.047). Although the length of ward and ICU stay was shorter in the NAC group than in controls, the difference was statistically insignificant (P = 0.598 and P = 0.629, respectively). Mortality, on the other hand, was 75% in the control group and 50% in the NAC group, with no statistically significant difference (P = 0.102). Concerning the change in the study parameters, only the decrease in diastolic blood pressure (DBP) was significantly higher with NAC (P = 0.042). The intubation and mechanical ventilation rates were higher, while oxygen with mask and nasal oxygen rates were lower with NAC, but the difference was statistically insignificant. Conclusions: Based on the current research, NAC is related to a significant decrease in RR, D-dimer, and DBP in severe COVID-19. Also, LDH was significantly lower in the NAC group than in the controls. More research with larger sample sizes is needed to validate the current study results.
Authors' Contribution: A. R and M. K. J. contributed to the conception and design. Other authors contributed to data collection and manuscript drafting. M. K. J. supervised the study. All authors approved the final version of the manuscript.
Clinical Trial Registration Code: This study is registered in the IRCT with the code IRCT20200509047364N3 (link: www.irct.ir/trial/54372 ).
Conflict of Interests: The authors have no conflict of interests.
Data Reproducibility: The dataset presented in the study is available on request from the corresponding author during submission or after its publication. The data are not publicly available since this is one of the new attempts to treat ICU-admitted COVID-19 patients. Ethical Approval: This study is approved under the ethical approval code of IR.HUMS.REC.1399.539 (link: ethics.research.ac.ir/form/n12u8iqoje51gyss.pdf). Funding/Support: No funding/support was received.
Informed Consent: Written informed consent was signed by all participants.
References
Assimakopoulos, Aretha, Komninos, Dimitropoulou, Lagadinou et al., N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study, Infect Dis (Lond), doi:10.1080/23744235.2021.1945675
Bhattacharya, Mondal, Naiya, Lyngdoh, Mukherjee et al., The beneficial role of N-acetylcysteine as an adjunctive drug in treatment of COVID-19 patients in a tertiary care hospital in India: an observational study, Int J Res Med Sci, doi:10.18203/2320-6012.ijrms20204010
Bunyavanich, Do, Vicencio, Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults, JAMA, doi:10.1001/jama.2020.8707
Cazzola, Calzetta, Page, Rogliani, Matera, Thiol-Based Drugs in Pulmonary Medicine: Much More than Mucolytics, Trends Pharmacol Sci, doi:10.1016/j.tips.2019.04.015
De Alencar, Moreira, Muller, Chaves, Fukuhara et al., Double-blind, Randomized, Placebo-controlled Trial With N-acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19), Clin Infect Dis, doi:10.1093/cid/ciaa1443
Dikalov, Nazarewicz, Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease, Antioxid Redox Signal, doi:10.1089/ars.2012.4604
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Ehre, Rushton, Wang, Hothem, Morrison et al., An Improved Inhaled Mucolytic to Treat Airway Mucoobstructive Diseases, Am J Respir Crit Care Med, doi:10.1164/rccm.201802-0245OC
Ershad, Naji, Vearrier, N Acetylcysteine. Treasure Island
Fisher, Curry, Evaluation and treatment of acetaminophen toxicity, Adv Pharmacol, doi:10.1016/bs.apha.2018.12.004
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Ibrahim, Smith, Lewis, Kon, Goldenberg, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol, doi:10.1016/j.clim.2020.108544
Laurindo, De Souza, Mde, Janiszewski, Redox aspects of vascular response to injury, Methods Enzymol, doi:10.1016/s0076-6879(02)52039-5
Lee, Hynan, Rossaro, Fontana, Stravitz et al., Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure, Gastroenterology, doi:10.1053/j.gastro.2009.06.006
Lu, Ma, He, Li, Zhu et al., N-acetylcysteine for adults with acute respiratory distress syndrome: A meta-analysis of randomized controlled trials, Hong Kong J Emerg Med, doi:10.1177/1024907918794559
Oliveira, Laurindo, Implications of plasma thiol redox in disease, Clin Sci (Lond), doi:10.1042/CS20180157
Puyo, Kreig, Saddi, Ansari, Prince, Case Report: Use of hydroxychloroquine and N-acetylcysteine for treatment of a COVID-19 patient, Research, doi:10.12688/f1000research.23995.2
Qi, He, Yang, Wang, Ma et al., Severity-associated markers and assessment model for predicting the severity of COVID-19: a retrospective study in Hangzhou, China, BMC Infect Dis, doi:10.1186/s12879-021-06509-6
Rahimi, Samimagham, Azad, Hooshyar, Arabi et al., The efficacy of N-Acetylcysteine in severe COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-021-05242-4
Redza-Dutordoir, Da, Activation of apoptosis signalling pathways by reactive oxygen species, Biochim Biophys Acta, doi:10.1016/j.bbamcr.2016.09.012
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Samimagham, Arabi, Hooshyar, Kazemijahromi, The Association of Non-Steroidal Anti-Inflammatory Drugs with COVID-19 Severity and Mortality, Arch Clin Infect Dis, doi:10.5812/archcid.106847
Samimagham, Azad, Arabi, Hooshyar, Sheikhtaheri et al., COVID-19 Severity and Comorbidities in Diabetic Patients, Dis Diagn, doi:10.34172/ddj.2021.18
Samimagham, Seddighi, Daryanavard, Jahromi, Recurrence of COVID-19 Infection, Shiraz E-Med J, doi:10.5812/semj.110656
Schettig, Sears, Klein, Tan-Lim, Aussems, COVID-19 Patient with Multifocal Pneumonia and Respiratory Difficulty Resolved Quickly: Possible Antiviral and Anti-Inflammatory Benefits of Quercinex (Nebulized Quercetin-NAC) as Adjuvant, Advances in Infectious Diseases, doi:10.4236/aid.2020.103006
Taher, Lashgari, Sedighi, Rahimi-Bashar, Poorolajal et al., A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome, Pharmacol Rep, doi:10.1007/s43440-021-00296-2
Tay, Poh, Renia, Macary, Ng, The trinity of COVID-19: immunity, inflammation and intervention, Nat Rev Immunol, doi:10.1038/s41577-020-0311-8
Touyz, Li, Delles, ACE2 the Janus-faced protein -from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19, Clin Sci (Lond), doi:10.1042/CS20200363
Wu, Hu, Zhang, Ren, Yu et al., Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients, Crit Care, doi:10.1186/s13054-020-03015-0
DOI record:
{
"DOI": "10.5812/jjnpp-129817",
"ISSN": [
"1735-7780",
"2228-7876"
],
"URL": "http://dx.doi.org/10.5812/jjnpp-129817",
"abstract": "<jats:p>Background: Today, various drugs have been investigated as the primary or complementary treatment for coronavirus disease 2019 (COVID-19). N-acetylcysteine (NAC) has been used as a mucolytic in pulmonary diseases. This drug apparently contributes to the retrieval of the intracellular antioxidant system. Objectives: This study aimed to determine the efficacy of NAC in severe COVID-19 patients admitted to the intensive care unit (ICU). Methods: This single-blinded randomized controlled phase III clinical trial included 40 patients with confirmed COVID-19 (based on polymerase chain reaction) admitted to the Shahid Mohammadi Hospital’s ICU, Bandar Abbas, Iran, in 2020. All cases had severe COVID-19. They were allocated randomly to two equal groups. Patients in the control group received standard drug therapy based on the treatment protocol of the national COVID-19 committee, while those in the NAC group received a single dose of intravenous NAC (300 mg/kg) upon admission to the ICU in addition to standard drug treatment. Clinical status and laboratory tests were done on admission to the ICU and then 14 days later or at discharge without knowing the patient grouping. Results: The two groups were comparable regarding age, gender, and other baseline laboratory and clinical parameters. At the final evaluation, respiratory rate (21.25 ± 4.67 vs. 27.37 ± 6.99 /min) and D-dimer (186.37 ± 410.23 vs. 1339.04 ± 2183.87 ng/mL) were significantly lower in the NAC group (P = 0.004 and P = 0.030, respectively). Also, a lower percentage of patients in the NAC group had lactate dehydrogenase (LDH) ≤ 245 U/L (0% vs. 25%, P = 0.047). Although the length of ward and ICU stay was shorter in the NAC group than in controls, the difference was statistically insignificant (P = 0.598 and P = 0.629, respectively). Mortality, on the other hand, was 75% in the control group and 50% in the NAC group, with no statistically significant difference (P = 0.102). Concerning the change in the study parameters, only the decrease in diastolic blood pressure (DBP) was significantly higher with NAC (P = 0.042). The intubation and mechanical ventilation rates were higher, while oxygen with mask and nasal oxygen rates were lower with NAC, but the difference was statistically insignificant. Conclusions: Based on the current research, NAC is related to a significant decrease in RR, D-dimer, and DBP in severe COVID-19. Also, LDH was significantly lower in the NAC group than in the controls. More research with larger sample sizes is needed to validate the current study results.</jats:p>",
"alternative-id": [
"c87f63dd101c5cbe2eb1093e8588b2b5ec847caf"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4313-7352",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rahimi",
"given": "Arash",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6636-198X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Samimagham",
"given": "HamidReza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hajiabdolrrasouli",
"given": "Ladan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassani Azad",
"given": "Mehdi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2345-8642",
"affiliation": [],
"authenticated-orcid": false,
"family": "Salimi Asl",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khajavi Mayvan",
"given": "Fatemeh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8520-2709",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boushehri",
"given": "Elham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arabi",
"given": "Mohsen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pazhoohesh",
"given": "Sepideh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9127-3594",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kazemi Jahromi",
"given": "Mitra",
"sequence": "additional"
}
],
"container-title": "Jundishapur Journal of Natural Pharmaceutical Products",
"container-title-short": "Jundishapur J Nat Pharm Prod",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"brieflands.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
19
]
],
"date-time": "2023-03-19T15:19:21Z",
"timestamp": 1679239161000
},
"deposited": {
"date-parts": [
[
2023,
3,
19
]
],
"date-time": "2023-03-19T15:19:25Z",
"timestamp": 1679239165000
},
"indexed": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T20:59:31Z",
"timestamp": 1704401971080
},
"is-referenced-by-count": 1,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1,
13
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
1,
13
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2013,
1,
1
]
],
"date-time": "2013-01-01T00:00:00Z",
"timestamp": 1356998400000
}
}
],
"link": [
{
"URL": "https://brieflands.com/articles/jjnpp-129817.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://brieflands.com/articles/jjnpp-129817.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3819",
"original-title": [],
"prefix": "10.5812",
"published": {
"date-parts": [
[
2023,
1,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
13
]
]
},
"publisher": "Briefland",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "key-A129817REF1-1"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "publisher",
"key": "key-A129817REF2-2"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "key-A129817REF3-3"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"doi-asserted-by": "publisher",
"key": "key-A129817REF4-4"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "key-A129817REF5-5"
},
{
"DOI": "10.1042/CS20200363",
"doi-asserted-by": "publisher",
"key": "key-A129817REF6-6"
},
{
"DOI": "10.1186/s13054-020-03015-0",
"doi-asserted-by": "publisher",
"key": "key-A129817REF7-7"
},
{
"DOI": "10.34172/ddj.2021.18",
"doi-asserted-by": "publisher",
"key": "key-A129817REF8-8"
},
{
"DOI": "10.5812/semj.110656",
"doi-asserted-by": "publisher",
"key": "key-A129817REF9-9"
},
{
"DOI": "10.5812/archcid.106847",
"doi-asserted-by": "publisher",
"key": "key-A129817REF10-10"
},
{
"DOI": "10.1001/jama.2020.8707",
"doi-asserted-by": "publisher",
"key": "key-A129817REF11-11"
},
{
"DOI": "10.1016/s0076-6879(02)52039-5",
"doi-asserted-by": "publisher",
"key": "key-A129817REF12-12"
},
{
"DOI": "10.1089/ars.2012.4604",
"doi-asserted-by": "publisher",
"key": "key-A129817REF13-13"
},
{
"DOI": "10.1016/j.bbamcr.2016.09.012",
"doi-asserted-by": "publisher",
"key": "key-A129817REF14-14"
},
{
"DOI": "10.1164/rccm.201802-0245OC",
"doi-asserted-by": "publisher",
"key": "key-A129817REF15-15"
},
{
"DOI": "10.1053/j.gastro.2009.06.006",
"doi-asserted-by": "publisher",
"key": "key-A129817REF16-16"
},
{
"DOI": "10.1016/bs.apha.2018.12.004",
"doi-asserted-by": "publisher",
"key": "key-A129817REF17-17"
},
{
"DOI": "10.1016/j.tips.2019.04.015",
"doi-asserted-by": "publisher",
"key": "key-A129817REF18-18"
},
{
"author": "Ershad M",
"journal-title": "N Acetylcysteine.",
"key": "key-A129817REF19-19",
"year": "2022"
},
{
"DOI": "10.1042/CS20180157",
"doi-asserted-by": "publisher",
"key": "key-A129817REF20-20"
},
{
"DOI": "10.1186/s12879-021-06509-6",
"doi-asserted-by": "publisher",
"key": "key-A129817REF21-21"
},
{
"DOI": "10.1186/s13063-021-05242-4",
"doi-asserted-by": "publisher",
"key": "key-A129817REF22-22"
},
{
"DOI": "10.1093/cid/ciaa1443",
"doi-asserted-by": "publisher",
"key": "key-A129817REF23-23"
},
{
"DOI": "10.18203/2320-6012.ijrms20204010",
"doi-asserted-by": "publisher",
"key": "key-A129817REF24-24"
},
{
"DOI": "10.1007/s43440-021-00296-2",
"doi-asserted-by": "publisher",
"key": "key-A129817REF25-25"
},
{
"DOI": "10.1080/23744235.2021.1945675",
"doi-asserted-by": "publisher",
"key": "key-A129817REF26-26"
},
{
"DOI": "10.4236/aid.2020.103006",
"doi-asserted-by": "publisher",
"key": "key-A129817REF27-27"
},
{
"DOI": "10.12688/f1000research.23995.2",
"doi-asserted-by": "publisher",
"key": "key-A129817REF28-28"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"doi-asserted-by": "publisher",
"key": "key-A129817REF29-29"
},
{
"DOI": "10.1177/1024907918794559",
"doi-asserted-by": "publisher",
"key": "key-A129817REF30-30"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://brieflands.com/articles/jjnpp-129817.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics"
],
"subtitle": [],
"title": "Efficacy of N-acetyl Cysteine in Severe COVID-19 Patients: A Randomized Controlled Phase III Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.5812/crossmark_update_policy",
"volume": "18"
}
