N-acetylcysteine for the treatment of COVID-19 among hospitalized patients
et al., Journal of Infection, doi:10.1016/j.jinf.2021.07.003, Jul 2021
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
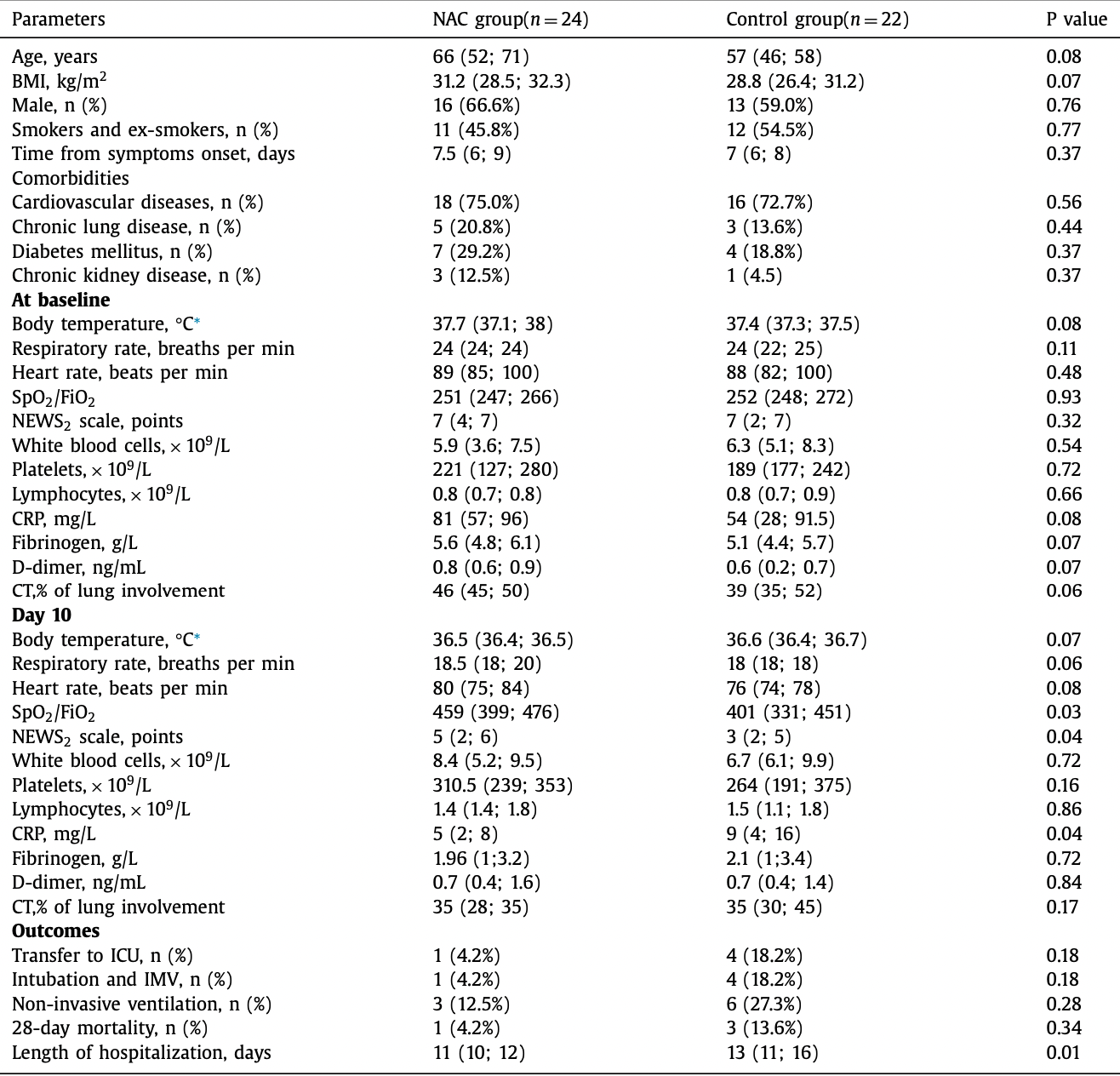
Prospective study of 24 hospitalized COVID-19 patients in Russia treated with NAC, and 22 matched controls, showing significantly improved SpO2/FiO2, and significantly shorter hospitalization with treatment.
|
risk of death, 69.4% lower, RR 0.31, p = 0.34, treatment 1 of 24 (4.2%), control 3 of 22 (13.6%), NNT 11.
|
|
risk of mechanical ventilation, 77.1% lower, RR 0.23, p = 0.18, treatment 1 of 24 (4.2%), control 4 of 22 (18.2%), NNT 7.1.
|
|
risk of ICU admission, 77.1% lower, RR 0.23, p = 0.18, treatment 1 of 24 (4.2%), control 4 of 22 (18.2%), NNT 7.1.
|
|
hospitalization time, 15.4% lower, relative time 0.85, p = 0.01, treatment 24, control 22.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Avdeev et al., 9 Jul 2021, retrospective, Russia, peer-reviewed, 4 authors, study period 12 April, 2020 - 20 June, 2020, average treatment delay 7.2 days.
Limited protection against SARS-CoV-2 infection and virus transmission after mRNA vaccination
Journal of Infection, doi:10.1016/j.jinf.2021.06.023
Predominance of delta variant among the COVID-19 vaccinated and unvaccinated individuals, India, May 2021 Dear Sir, * p = 0.003 for the proportions with severe disease among fully vaccinated and unvaccinated individuals. * * p value (1-tail) = 0.018 for the proportions of deaths among fully vaccinated and unvaccinated individuals. * * * p value (1-tail) = 0.046 for the proportions of deaths among partially vaccinated and unvaccinated individuals.
Ethical statement This research was approved by the University of Campus Biomedico Ethics Review Committee (Approval number 8.1(21).21 OSS).
Declaration of Competing Interest The authors declare no competing interests.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jinf.2021.06.026 .
Ethical approval The study was approved by the Medical Ethical Committee of Sechenov Moscow Medical University (protocol number 08-20/1).
CRediT authorship contribution statement Sergey
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.07.032 . In our prospective cohort study, which included 1958 HCWs vaccinated with the BNT162b2 mRNA vaccine between January 1 and March 30, 2021, 22 HCWs were infected with SARS-CoV-2 ≤ 14 days after the first vaccine dose and had the second dose postponed > 2 months. The anti-SARS-CoV-2 antibody response in this group of HCWs (group A: concomitant infection) was compared with that observed in other groups: i.e., HCWs who got infected from March 2020 to November 2020 and were vaccinated in January 2021 (group B: prior infection, ≥ 2 months, n = 55); HCWs who got infected in December 2020 and had vaccination postponed > 1 month (group C: prior infection, < 2 months, n = 26), and naïve HCWs, who were regularly vaccinated in January 2021 (group D: naïve, n = 55). Group A..
References
Adorni, Prinelli, Bianchi, Giacomelli, Pagani et al., Self-reported symptoms of SARS-CoV-2 infection in a nonhospitalized population in Italy: cross-sectional study of the EPICOVID19 web-based survey, JMIR Public Health Surveill
Alamdari, Moghaddam, Amini, Keramati, Zarmehri et al., Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial, Eur J Pharmacol, doi:10.1016/j.ejphar.2020.173494
Aldini, Altomare, Baron, Vistoli, Carini et al., N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why, Free Radic Res, doi:10.1080/10715762.2018.1468564
Anichini, Terrosi, Gandolfo, Savellini, Fabrizi et al., SARS-CoV-2 antibody response in persons with past natural infection, N Engl J Med
Anichini, Terrosi, Gandolfo, Savellini, Fabrizi et al., SARS-CoV-2 antibody response in persons with past natural infection, N Engl J Med
Bae, Lee, Lim, Lee, Lim et al., Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea, J Korean Med Sci
Bastos, Tavaziva, Abidi, Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis, BMJ
Bell, Trouble in Testing Land
Betton, Livrozet, Planas, Fayol, Monel et al., Sera neutralizing activities against SARS-CoV-2 and multiple variants six month after hospitalization for COVID-19, Clin Infect Dis, doi:10.1093/cid/ciab308
Bhoyar, Jain, Sehgal, Divakar, Sharma et al., High throughput detection and genetic epidemiology of SARS-CoV-2 using COVID-Seq next-generation sequencing, PLoS One, doi:10.1371/journal.pone.0247115
Boccuto, Dragoni, Department, Sacco ; Sansone, Tiraboschi et al., Effectiveness of BNT162b2 vaccine against the B.1.1.7 variant of SARS-CoV-2 among healthcare workers in Brescia, Italy, J Infect, doi:10.1016/j.jinf.2021.04.038
Bourgonje, Offringa, Van Eijk, Abdulle, Hillebrands et al., N-Acetylcysteine and hydrogen sulfide in Coronavirus disease 2019, Antioxid Redox Signal, doi:10.1089/ars.2020.8247
Buonfrate, Piubelli, Gobbi, Martini, Bertoli et al., Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: a prospective study, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.07.024
Capetti, Stangalini, Borgonovo, Mileto, Oreni et al., Impressive boosting of anti-S1/S2 IgG production in COVID-19-experienced patients after the first shot of the BNT162b2 mRNA COVID-19 Vaccine, Clin Infect Dis
Chevallier, Coste-Burel, Bourgeois, Peterlin, Garnier et al., Safety and immunogenicity of a first dose of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic stem-cells recipients, EJHaem, doi:10.1002/jha2.242
Cleemput, Dumon, Fonseca, Karim, Giovanetti et al., Genome detective coronavirus typing tool for rapid identification and characterization of novel coronavirus genomes, Bioinformatics, doi:10.1093/bioinformatics/btaa145
Cosentino, BPO-BIOEPINE-Biogroup -Plateau technique Chocolaterie, Levallois
Dagan, Barda, Kepten, Miron, Perchik et al., BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting, N Engl J Med
Davies, Abbott, Barnard, Jarvis, Kucharski et al., CMMID COVID-19
Davies, Abbott, Barnard, Jarvis, Kucharski et al., Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England, Science
Davies, Jarvis, Edmunds, Jewell, Diaz-Ordaz et al., Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7, Nature, doi:10.1038/s41586-021-03426-1
De Alencar, Moreira, Müller, Chaves, Fukuhara et al., Double-blind, randomized, placebo-controlled trial with Nacetylcysteine for treatment of severe acute respiratory syndrome caused by COVID-19, Clin Infect Dis, doi:10.1093/cid/ciaa1443
Dhar, Marwal, Radhakrishnan, Ponnusamy, Jolly et al., Genomic characterization and Epidemiology of an emerging SARS-CoV-2 variant in Delhi, doi:10.1101/2021.06.02.21258076
Dimeglio, Herin, Miedougé, Da-Silva, Porcheron et al., One year later: SARS-CoV-2 immune response and vaccination of healthcare workers post-infection, J Infect, doi:10.1016/j.jinf.2021.06.016
Doria-Rose, Suthar, Makowski, O'connell, Mcdermott et al., Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19, N Engl J Med
Doria-Rose, Suthar, Makowski, O'connell, Mcdermott et al., mRNA-1273 Study Group. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19, N Engl J Med
Falsey, Respiratory syncytial virus infection in older persons, Vaccine
Fantini, Yahi, Azzaz, Chahinian, Structural dynamics of SARS-CoV-2 variants: a health monitoring strategy for anticipating COVID-19 outbreaks, J Infect, doi:10.1016/j.jinf.2021.06.001
Faria, Mellan, Whittaker, Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Braz Sci, doi:10.1126/science.abh2644
Favresse, Bayart, Mullier, Dogne, Closset et al., Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2), Clin Microbiol Infect
Favresse, Bayart, Mullier, Elsen, Eucher et al., Antibody titers decline 3-month post-vaccination with BNT612b2. 202, Emerg Microbes Infect, doi:10.1080/22221751.2021.1953403
Favresse, Bayart, Mullier, Elsen, Eucher et al., Antibody titers decline 3-month post-vaccination with BNT612b2. 202, Emerg Microbes Infect, doi:10.1080/22221751.2021.1953403
Favresse, Douxfils, Importance of sample dilution in the evaluation of the antibody response after SARS-CoV-2 vaccination, J Infect, doi:10.1016/j.jinf.2021.07.001
Gaymard, Bosetti, Feri, Destras, Enouf et al., Early assessment of diffusion and possible expansion of SARS-CoV-2 lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, Eurosurveillance
Gerhards, Thiaucourt, Kittel, Becker, Ast et al., Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection, Int J Infect Dis
Gobbi, Buonfrate, Moro, Rodari, Piubelli et al., Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection, Viruses, doi:10.3390/v13030422
Group, Diaz-Ordaz, Keogh, Eggo, Funk et al., Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England, Science, doi:10.1126/science.abg3055
Hacisuleyman, Hale, Saito, Blachere, Bergh et al., Vaccine breakthrough infections with SARS-CoV-2 variants, N Engl J Med, doi:10.1056/NEJMoa2105000
Hasan, Rabbani, Anam, Huq, Secukinumab in severe COVID-19 pneumonia: does it have a clinical impact?, J Infect, doi:10.1016/j.jinf.2021.05.011
Hay, Kennedy-Shaffer, Kanjilal, Lennon, Gabriel et al., Estimating epidemiologic dynamics from cross-sectional viral load distributions, Science
Henry, Szergyuk, Santos De Oliveira, Lippi, Juszczyk et al., Utility of google trends in anticipating COVID-19 outbreaks in Poland, Pol Arch Intern Med
Ibrahim, Perl, Smith, Lewis, Kon et al., Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous Nacetylcysteine, Clin Immunol, doi:10.1016/j.clim.2020.108544
Janik, Niemcewicz, Podogrocki, Majsterek, Bijak, The Emerging Concern and Interest SARS CoV-2 Variants, Pathogens
Karim, De Oliveira, New SARS-CoV-2 variants -clinical, public health, and vaccine implications, N Engl J Med
Kawasaki, Kawai, Akira, Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity, Immunol Rev
Khoury, Cromer, Reynaldi, Schlub, Wheatley et al., Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection, Nat Med, doi:10.1038/s41591-021-01377-8
Kissler, Fauver, Mack, Tai, Breban et al., Densely sampled viral trajectories suggest longer duration of acute infection with B.1.1.7 variant relative to non-B.1.1.7 SARS-CoV-2, medRxiv
Krammer, A correlate of protection for SARS-CoV-2 vaccines is urgently needed, Nat Med, doi:10.1038/s41591-021-01432-4
Krammer, Srivastava, Alshammary, Amoako, Awawda et al., Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine, N Engl J Med
Krammer, Srivastava, Alshammary, Amoako, Awawda et al., Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine, N Engl J Med, doi:10.1056/NEJMc2101667
Li, Yi, Luo, Development and Clinical Application of A rapid Ig-M-IgG combined antibody test for SARS-CoV-2 infection diagnosis, J Med Virol
Lippi, Mattiuzzi, Cervellin, Google search volume predicts the emergence of COVID-19 outbreaks, Acta Biomed
Liu, Liu, Xia, Zhang, Fontes-Garfias et al., Neutralizing activity of BNT162b2-elicited serum, N Engl J Med
Liu, Liu, Xia, Zou, Weaver et al., BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants, Nature
Liu, Liu, Xia, Zou, Weaver et al., BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants, Nature
Lustig, Sapir, Regev-Yochay, Cohen, Fluss et al., BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers, doi:10.1016/S2213-2600(21)00220-4
Lustig, Zuckerman, Nemet, Atari, Kliker et al., Neutralising capacity against delta (B.1.617.2) and other variants of concern following comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel, Euro Surveill, doi:10.2807/1560-7917.ES.2021.26.26.2100557
Malani, Ramachandran, Tandel, Parasa, Sudharshini et al., SARS-CoV-2 seroprevalence in Tamil Nadu in October, doi:MedRxiv2021:2021.02.03.21250949.10.1101/2021.02.03.21250949
Manisty, Otter, Treibel, Mcknight, Altmann et al., Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals, Lancet
Marks, Millat-Martinez, Ouchi, Roberts, Alemany et al., Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30985-3
Meyer, Buhl, Kampf, Magnussen, Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals, Am J Respir Crit Care Med, doi:10.1164/ajrccm.152.3.7663783
Minh, Schmidt, Chernomor, Schrempf, Woodhams et al., IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic Era, Mol Biol Evol, doi:10.1093/molbev/msaa015ignorespacesMedline
Mohindra, Pinnaka, Vaccine breakthrough infections with SARS-CoV-2 variants, N Engl J Med, doi:10.1056/NEJMc2107808
Moser, Leo, Key concepts in immunology, Vaccine
Mueller, Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer, Clin Chim Acta
Nakamura, Yamada, Tomii, Katoh, Parallelization of MAFFT for large-scale multiple sequence alignments, Bioinformatics
Nikolich-Zugich, Knox, Rios, Natt, Bhattacharya et al., SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes, GeroScience
Park, Cha, Suh, Jung, Department et al., Emergence of a new SARS-CoV-2 variant in the UK, J Infect
Pereira, Tosta, Lima, Reboredo De Oliveira Da Silva, Nardy et al., Genomic surveillance activities unveil the introduction of the SARS-CoV-2 B1.525 variant of interest in Brazil: case report, J Med Virol
Planas, Bruel, Grzelak, Guivel-Benhassine, Staropoli et al., Sensitivity of infectious SARS-CoV-2 B1.1.7 and B.1.351 variants to neutralizing antibodies, Nat Med
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of infectious SARS-CoV-2 variant B.1.617.2 to monoclonal antibodies and sera from convalescent and vaccinated individuals, doi:BioRxiv2021:2021.05.26.445838.10.1101/2021.05.26.445838
Prendecki, Clarke, Brown, Cox, Gleeson et al., Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine, Lancet
Prendecki, Clarke, Brown, Cox, Gleeson et al., Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine, Lancet
Prévost, Finzi, The great escape? SARS-CoV-2 variants evading neutralizing responses, Cell Host Microbe, doi:10.1016/j.chom.2021.02.010
Rambaut, Loman, Pybus, Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations
Renieris, Katrini, Damoulari, Akinosoglou, Psarrakis et al., Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus, Shock, doi:10.1097/SHK.0000000000001562
Reuters, Denmark to send back inaccurate antibody tests from China's Livzon
Saadat, Tehrani, Logue, Newman, Frieman et al., Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2, JAMA
Salvagno, Henry, Di Piazza, Pighi, De Nitto et al., Anti-SARS-CoV-2 receptor-binding domain total antibodies response in seropositive and seronegative healthcare workers undergoing COVID-19 mRNA BNT162b2 vaccination, Diagnostics, doi:10.3390/diagnostics11050832
Singh, Rophina, Chaudhry, Vigneshwar, Bala et al., Variants of concern responsible for SARS-CoV-2 vaccine breakthrough infections from India, OSF Pre, doi:10.31219/osf.io/fgd4x
Smith, Jefferies, Role of DNA/RNA sensors and contribution to autoimmunity, Cytokine Growth Factor Rev
Spf, COVID-19 : point épidémiologique du 18 mars 2021
Stang, Robers, Schonert, Jöckel, Spelsberg et al., The performance of the SARS-CoV-2 RT-PCR test as a tool for detecting SARS-CoV-2 infection in the population, J Infect
Suhandynata, Bevins, Tran, Huang, Hoffman et al., SARS-CoV-2 Serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses, J Appl Lab Med, doi:10.1093/jalm/jfab080
Tang, Toovey, Harvey, Hui, Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK, J Infect
Tegally, Wilkinson, Giovanetti, Detection of a SARS-CoV-2 variant of concern in South Africa, Nature, doi:10.1038/s41586-021-03402-9
Tiraboschi, Lombardo, Castelli, Effectiveness of BNT162b2 vaccine against the B.1.1.7 variant of SARS-CoV-2 among healthcare workers in Brescia, Italy, J Infect
Tober-Lau, Schwarz, Hillus, Spieckermann, Helbig et al., Outbreak of SARS-CoV-2 B1.1.7 lineage after vaccination in long-term care facility, Germany, February-March 2021, Emerg Infect Dis J
Tre-Hardy, Wilmet, Beukinga, Dogne, Douxfils et al., RECOV-ERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19, Clin Chem Lab Med: CCLM /FESCC, doi:10.1056/NEJMoa2021436
Tré-Hardy, Cupaiolo, Wilmet, Beukinga, Blairon, Waning antibodies in SARS-CoV-2 naïve vaccinees: results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers, J Infect
Tré-Hardy, Cupaiolo, Wilmet, Beukinga, Blairon, Waning antibodies in SARS-CoV-2 naïve vaccinees: results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers, J Infect
Tré-Hardy, Cupaiolo, Wilmet, Beukinga, Blairon, Waning antibodies in SARS-CoV-2 naïve vaccinees: results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers, J Infect, doi:10.1016/j.jinf.2021.06.017
Turner, O'halloran, Kalaidina, Kim, Schmitz et al., SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses, Online ahead of print, doi:10.1038/s41586-021-03738-2
Vaida, Liu, Fast implementation for normal mixed effects models with censored response, J Comput Graph Stat
Vancheeswaran, Willcox, Stuart, Accuracy of rapid point-ofcare antibody test in patients with suspected or confirmed COVID-19, medRxiv
Vicenti, Gatti, Scaggiante, Boccuto, Zago et al., Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection, Int J Infect Dis
Volz, Mishra, Chand, Barrett, Johnson et al., Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England, Nature, doi:10.1038/s41586-021-03470-x
Wang, Casner, Nair, Wang, Yu et al., Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization, Cell Host Microbe
Wang, Nair, Liu, Iketani, Luo et al., Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Widge, Rouphael, Jackson, Anderson, Roberts et al., Durability of responses after SARS-CoV-2 mRNA-1273 vaccination, N Engl J Med
Xie, Ding, Li, Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test, J Med Virol
Yadav, Sapkal, Ella, Sahay, Nyayanit et al., 2 with sera of COVID-19 recovered cases and vaccinees of BBV152, doi:BioRxiv2021:2021.06.05.447177.10.1101/2021.06.05.447177
Yoneyama, Fujita, Recognition of viral nucleic acids in innate immunity, Rev Med Virol
Zani, Caccuri, Messali, Bonfanti, Caruso, Serosurvey in BNT162b2 vaccine-elicited neutralizing antibodies against authentic B.1, Emerg Microbes Infect
DOI record:
{
"DOI": "10.1016/j.jinf.2021.07.003",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2021.07.003",
"alternative-id": [
"S0163445321003297"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "N-acetylcysteine for the treatment of COVID-19 among hospitalized patients"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2021.07.003"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The British Infection Association. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Avdeev",
"given": "Sergey N.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gaynitdinova",
"given": "Viliya V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Merzhoeva",
"given": "Zamira M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berikkhanov",
"given": "Zelimkhan G.-M.",
"sequence": "additional"
}
],
"container-title": [
"Journal of Infection"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"journalofinfection.com",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.fr",
"clinicalkey.com.au",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
10
]
],
"date-time": "2021-07-10T07:01:44Z",
"timestamp": 1625900504000
},
"deposited": {
"date-parts": [
[
2022,
1,
6
]
],
"date-time": "2022-01-06T10:28:23Z",
"timestamp": 1641464903000
},
"indexed": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T05:44:54Z",
"timestamp": 1641534294661
},
"is-referenced-by-count": 2,
"issn-type": [
{
"type": "print",
"value": "0163-4453"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445321003297?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445321003297?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "94-118",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2021.07.003_bib0001",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet",
"key": "10.1016/j.jinf.2021.07.003_bib0002",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2021.05.011",
"article-title": "Secukinumab in severe COVID-19 pneumonia: does it have a clinical impact?",
"author": "Hasan",
"doi-asserted-by": "crossref",
"first-page": "e11",
"issue": "1",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2021.07.003_bib0003",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1089/ars.2020.8247",
"article-title": "N-Acetylcysteine and hydrogen sulfide in Coronavirus disease 2019",
"author": "Bourgonje",
"doi-asserted-by": "crossref",
"journal-title": "Antioxid Redox Signal",
"key": "10.1016/j.jinf.2021.07.003_bib0004",
"year": "2021"
},
{
"DOI": "10.1097/SHK.0000000000001562",
"article-title": "Serum hydrogen sulfide and outcome association in pneumonia by the SARS-CoV-2 coronavirus",
"author": "Renieris",
"doi-asserted-by": "crossref",
"first-page": "633",
"issue": "5",
"journal-title": "Shock",
"key": "10.1016/j.jinf.2021.07.003_bib0005",
"volume": "54",
"year": "2020"
},
{
"DOI": "10.1164/ajrccm.152.3.7663783",
"article-title": "Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals",
"author": "Meyer",
"doi-asserted-by": "crossref",
"first-page": "1055",
"issue": "3",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/j.jinf.2021.07.003_bib0006",
"volume": "152",
"year": "1995"
},
{
"DOI": "10.1080/10715762.2018.1468564",
"article-title": "N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why",
"author": "Aldini",
"doi-asserted-by": "crossref",
"first-page": "751",
"issue": "7",
"journal-title": "Free Radic Res",
"key": "10.1016/j.jinf.2021.07.003_bib0007",
"volume": "52",
"year": "2018"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"article-title": "Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine",
"author": "Ibrahim",
"doi-asserted-by": "crossref",
"journal-title": "Clin Immunol",
"key": "10.1016/j.jinf.2021.07.003_bib0008",
"volume": "219",
"year": "2020"
},
{
"DOI": "10.1016/j.ejphar.2020.173494",
"article-title": "Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial",
"author": "Alamdari",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Pharmacol",
"key": "10.1016/j.jinf.2021.07.003_bib0009",
"volume": "885",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1443",
"article-title": "Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by COVID-19",
"author": "de Alencar",
"doi-asserted-by": "crossref",
"first-page": "e736",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jinf.2021.07.003_bib0010",
"volume": "72",
"year": "2021"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"score": 1,
"short-container-title": [
"Journal of Infection"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"N-acetylcysteine for the treatment of COVID-19 among hospitalized patients"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "84"
}
