Impact of N-Acetylcysteine on Modulating Inflammation in Patients Hospitalized with Moderate COVID-19 Infections: A Prospective Randomized Trial
et al., Archives of Pharmaceutical Sciences Ain Shams University, doi:10.21608/aps.2023.212265.1122, NCT04792021, Jun 2023
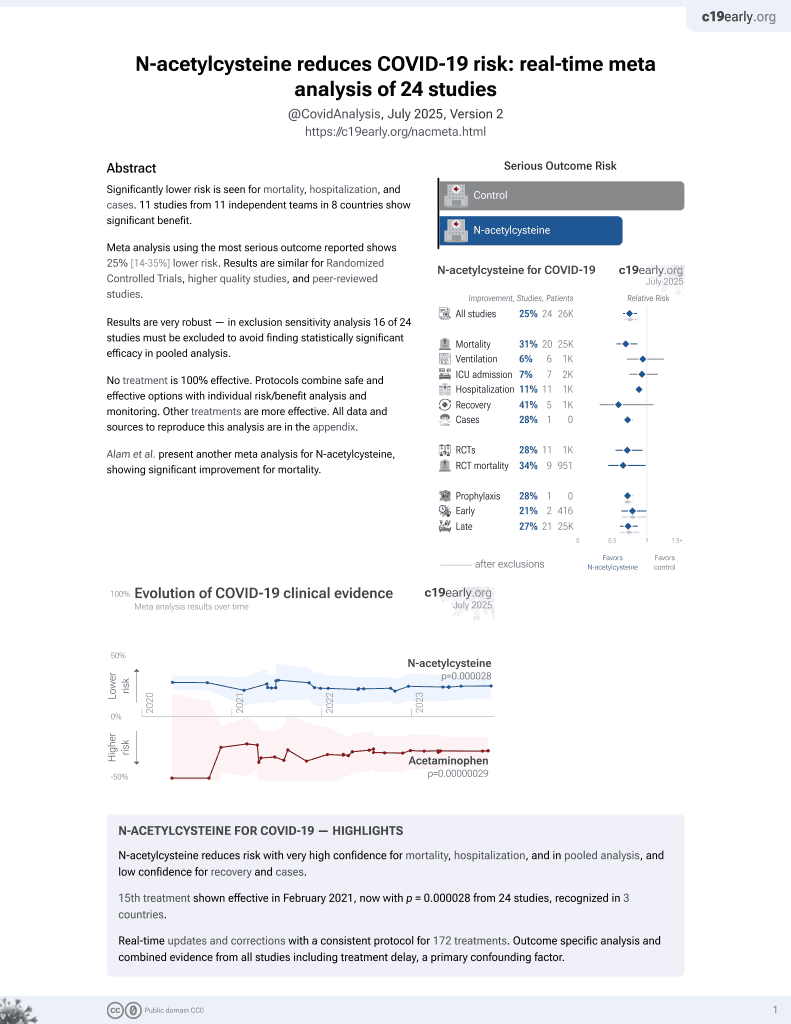
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 60 hospitalized patients showing that oral N-acetylcysteine (NAC) at 1800mg daily significantly decreased plasma TNF-α levels and increased glutathione peroxidase levels. The NAC group had a shorter duration of oxygen support, while there were no significant difference for length of hospital stay, need for oxygen support, or mortality. Overall, the addition of high-dose NAC reduced inflammatory markers and oxidative stress in moderate COVID-19.
Limitations include the small sample size, late treatment, lack of blinding, potential overlap of treatment effect with SOC, clinical significance of biomarker results, and limited adverse event reporting.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 1 of 30 (3.3%), control 1 of 30 (3.3%).
|
|
risk of oxygen therapy, 15.0% lower, RR 0.85, p = 0.60, treatment 17 of 30 (56.7%), control 20 of 30 (66.7%), NNT 10.
|
|
oxygen time, 33.3% lower, relative time 0.67, p = 0.005, treatment 30, control 30.
|
|
hospitalization time, 12.5% lower, relative time 0.88, p = 0.45, treatment 30, control 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sherkawy et al., 1 Jun 2023, Randomized Controlled Trial, Egypt, peer-reviewed, 5 authors, study period March 2021 - April 2022, trial NCT04792021 (history).
Contact: lamia.elwakeel@pharma.asu.edu.eg.
Impact of N-Acetylcysteine on Modulating Inflammation in Patients Hospitalized with Moderate COVID-19 Infections: A Prospective Randomized Trial
Archives of Pharmaceutical Sciences Ain Shams University, doi:10.21608/aps.2023.212265.1122
N-acetylcysteine (NAC) is a widely used safe mucolytic, that demonstrated positive impacts on various respiratory diseases via its anti-inflammatory and antioxidant effects. The study aimed to evaluate the potential benefit of adding high-dose oral N-acetylcysteine in hospitalized moderate-severity COVID-19 patients. A prospective, single-center, randomized clinical trial on 60 hospitalized moderate COVID-19 patients who were randomly assigned to the NAC group (30); received NAC daily at 1800 mg added to the institutional protocol, or non-NAC group (30); received only the institutional protocol. Outcomes. The primary outcome was the change in plasma TNF-α, IL-6, and glutathione peroxidase levels. Secondary outcomes were the length of hospital stay, need for oxygen support, duration of oxygenation, and mortality rate between the two study groups. At the study end, a significant decline in TNF-α levels (p< 0.001) and a significant increase in glutathione peroxidase in the NACtreated group (p= 0.001) were evident. Groups were comparable in IL-6 levels (p= 0.810). The duration of oxygen support significantly decreased in the NAC group (p= 0.005). On the contrary, hospital stay length and oxygen support need was not affected by the addition of 0.45, 0.42, respectively). The mortality rate was comparable in both groups. In conclusion, the Addition of 1800 mg of NAC to the institutional treatment protocol for moderate COVID-19 patients has led to a decline in the levels of plasma TNF-α and increased glutathione peroxidase levels. Moreover, the duration required for oxygen support decreased in patients needing supplemental oxygenation.
Declarations
Ethics approval and consent to participate The study protocol was approved by the Scientific Research Ethics Committee, Faculty of Pharmacy, Ain Shams University (No. 128). Written informed consents were signed and collected from all the study participants.
Consent to publish All authors have read and agreed to the published version of the manuscript.
Competing interests The authors have no competing interests.
References
Andreou, Trantza, Filippou, Sipsas, Tsiodras, COVID-19: The Potential Role of Copper and N-acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments Against SARS-CoV-2, In Vivo, doi:10.21873/invivo.11946
Badawy Abdelfattah, El-Zahapy, Hospital Response to COVID-19, doi:10.14293/S2199-1006.1.SOR-.PPD4QZX.v1
Baj, Karakuła-Juchnowicz, Teresiński, Buszewicz, Ciesielka et al., COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge, J Clin Med, doi:10.3390/jcm9061753
Bhattacharya, Mondal, Naiya, Lyngdoh, Mukherjee et al., The beneficial role of N-acetylcysteine as an adjunctive drug in treatment of COVID-19 patients in a tertiary care hospital in India: an observational study, Int J Res Med Sci, doi:10.18203/2320-6012.ijrms
Calverley, Rogliani, Papi, Safety of N-Acetylcysteine at High Doses in Chronic Respiratory Diseases: A Review, Drug Saf, doi:10.1007/s40264-020-01026-y
Cheng, He, Clinical and Biochemical Potential of Antioxidants in Treating Polycystic Ovary Syndrome, Int J Womens Health, doi:10.2147/IJWH.S345853
Cucinotta, Vanelli, WHO Declares COVID-19 a Pandemic, Acta Biomed, doi:10.23750/abm.v91i1.9397
Dekhuijzen, Van Beurden, The role for N-acetylcysteine in the management of COPD, Int J Chron Obstruct Pulmon Dis, doi:10.2147/copd.2006.1.2.99
Devrim-Lanpir, Hill, Knechtle, How N-Acetylcysteine Supplementation Affects Redox Regulation, Especially at Mitohormesis and Sarcohormesis Level: Current Perspective. Antioxidants (Basel), doi:10.3390/antiox10020153
Dikalov, Nazarewicz, Angiotensin IIinduced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease, Antioxid Redox Signal, doi:10.1089/ars.2012.4604
Dodd, Dean, Copolov, Malhi, Berk, N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility, Expert Opin Biol Ther, doi:10.1517/14728220802517901
Dogra, Goyal, Sharma, Corona virus: A novel outbreak, Biomedical and Pharmacology Journal, doi:10.13005/bpj/1853
Doughan, Harrison, Dikalov, Molecular mechanisms of angiotensin IImediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction, Circulation Research, doi:10.1161/CIRCRESAHA.107.162800
Esakandari, Nabi-Afjadi, Fakkari-Afjadi, Farahmandian, Miresmaeili et al., A comprehensive review of COVID-19 characteristics, Biol Proced Online, doi:10.1186/s12575-020-00128-2
Faghfouri, Zarezadeh, Tavakoli-Rouzbehani, Radkhah, Faghfuri et al., The effects of Nacetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials, Eur J Pharmacol, doi:10.1016/j.ejphar.2020.173368
Flora, Balansky, Maestra, None, doi:10.1056/NEJMoa042976
Flora, Balansky, Maestra, Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19, FASEB J, doi:10.1096/fj.202001807
Flora, Grassi, Carati, Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment, Eur Respir J, doi:10.1183/09031936.97.10071535
Gennaro, Pizzol, Marotta, Antunes, Racalbuto et al., Coronavirus Diseases (COVID-19) Current Status and Future Perspectives: A Narrative Review, Int J Environ Res Public Health, doi:10.3390/ijerph17082690
Ghafouri-Fard, Noroozi, Omrani, Branicki, Pośpiech et al., Angiotensin converting enzyme: A review on expression profile and its association with human disorders with special focus on SARS-CoV-2 infection, Vascul Pharmacol, doi:10.1016/j.vph.2020.106680
Göbölös, Rácz, Hogan, Remsey-Semmelweis, Atallah et al., The role of renin-angiotensin system activated phagocytes in the SARS-CoV-2 coronavirus infection, J Vasc Surg, doi:10.1016/j.jvs.2020.12.056
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol, doi:10.1002/jmv.26232
Hussain Basha, Corona virus drugs-a brief overview of past, present and future, J. Peer Scientist, doi:10.5281/zenodo.3747641
Izquierdo, Soriano, González, Lumbreras, Ancochea et al., Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19, Sci Prog, doi:10.1177/00368504221074574
Kasal, Lorenzo, Tibiriçá, COVID-19 and Microvascular Disease: Pathophysiology of SARS-CoV-2 Infection With Focus on the Renin-Angiotensin System. Heart Lung Circ, doi:10.1016/j.hlc.2020.08.010
Khomich, Kochetkov, Bartosch, Ivanov, Redox Biology of Respiratory Viral Infections, Viruses, doi:10.3390/v10080392
Marco, Foti, Corsico, Where are we with the use of N-acetylcysteine as a preventive and adjuvant treatment for COVID-19?, Eur Rev Med Pharmacol Sci, doi:10.26355/eurrev_202201_27898
Mata, Morcillo, Gimeno, Cortijo, Nacetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV), Biochem Pharmacol, doi:10.1016/j.bcp.2011.05.014
Medina-Enríquez, Lopez-León, Escalante, Aponte-Torres, Cuapio et al., ACE2: the molecular doorway to SARS-CoV-2. Cell Biosci, doi:10.1186/s13578-020-00519-8
Mp, Nacetylcysteine as a potential treatment for COVID-19, Future Microbiol, doi:10.2217/fmb-2020-0074
Muhammad, Kani, Iliya, Muhammad, Binji et al., Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: A cross-sectional comparative study in Jigawa, Northwestern Nigeria, SAGE Open Med. Feb, doi:10.1177/2050312121991246
Oliver, Dean, Camfield, Blair-West, Ng et al., N-acetyl cysteine in the treatment of obsessive compulsive and related disorders: a systematic review, Clin Psychopharmacol Neurosci, doi:10.9758/cpn.2015.13.1.12
Parasher, COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment, Postgrad Med J, doi:10.1136/postgradmedj-2020-138577
Paschalis, Theodorou, Margaritelis, Kyparos, Nikolaidis, N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione, Free Radic Biol Med, doi:10.1016/j.freeradbiomed
Poppe, Wittig, Jurida, Bartkuhn, Wilhelm et al., The NF-κBdependent and -independent transcriptome and chromatin landscapes of human coronavirus 229E-infected cells, PLoS Pathog, doi:10.1371/journal.ppat.1006286
Pueyo, Gonzalez, Nicoletti, Savoie, Arnal et al., Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress, Arterioscler Thromb Vasc Biol, doi:10.1161/01.atv.20.3.645
References, None
Ruiz-Ortega, Lorenzo, Rupérez, König, Wittig et al., Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms, Circ Res, doi:10.1161/01.res.86.12.1266
Ruiz-Ortega, Ruperez, Lorenzo, Esteban, Blanco et al., Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney, Kidney Int Suppl, doi:10.1046/j.1523-1755.62.s82.4.x
Sadeghi-Haddad-Zavareh, Bayani, Shokri, Ebrahimpour, Babazadeh et al., C-Reactive Protein as a Prognostic Indicator in COVID-19 Patients, Interdiscip Perspect Infect Dis, doi:10.1155/2021/5557582
Shang, Pan, Yang, Zhong, Shang et al., Management of critically ill patients with COVID-19 in ICU: statement from frontline intensive care experts in Wuhan, Ann Intensive Care, doi:10.1186/s13613-020-00689-1
Sharafkhah, Abdolrazaghnejad, Zarinfar, Mohammadbeigi, Massoudifar et al., Safety and efficacy of N-acetylcysteine for prophylaxis of ventilatorassociated pneumonia: a randomized, double blind, placebo-controlled clinical trial, Med Gas Res, doi:10.4103/2045-9912.229599
Singhal, A Review of Coronavirus Disease-2019 (COVID-19), Indian J Pediatr, doi:10.1007/s12098-020-03263-6
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat Med, doi:10.1038/s41591-020-0868-6
Taher, Lashgari, Sedighi, Rahimi-Bashar, Poorolajal et al., A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome, Pharmacol Rep
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Zhang, Zhu, Wang, Yang, Liu et al., Characterizing COVID-19 transmission: Incubation period, reproduction rate, and multiple-generation spreading, Front Phys, doi:10.3389/fphy.2020.589963
DOI record:
{
"DOI": "10.21608/aps.2023.212265.1122",
"ISSN": [
"2356-8399"
],
"URL": "http://dx.doi.org/10.21608/aps.2023.212265.1122",
"author": [
{
"affiliation": [],
"family": "Sherkawy",
"given": "Sara",
"sequence": "first"
},
{
"affiliation": [],
"family": "Elwakeel",
"given": "Lamia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaalan",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moharram",
"given": "Ayman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abouelwafa",
"given": "Mohamed",
"sequence": "additional"
}
],
"container-title": "Archives of Pharmaceutical Sciences Ain Shams University",
"container-title-short": "Archives of Pharmaceutical Sciences Ain Shams University",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"aps.journals.ekb.eg"
]
},
"created": {
"date-parts": [
[
2023,
7,
17
]
],
"date-time": "2023-07-17T18:09:29Z",
"timestamp": 1689617369000
},
"deposited": {
"date-parts": [
[
2023,
7,
17
]
],
"date-time": "2023-07-17T18:19:19Z",
"timestamp": 1689617959000
},
"indexed": {
"date-parts": [
[
2023,
7,
18
]
],
"date-time": "2023-07-18T04:29:45Z",
"timestamp": 1689654585069
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
6,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
6,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://aps.journals.ekb.eg/article_306468_420dd7e7231b0a5b2177d63fb6d2c8c7.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9060",
"original-title": [],
"page": "129-146",
"prefix": "10.21608",
"published": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
6,
1
]
]
},
"publisher": "Egypts Presidential Specialized Council for Education and Scientific Research",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://aps.journals.ekb.eg/article_306468.html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Impact of N-Acetylcysteine on Modulating Inflammation in Patients Hospitalized with Moderate COVID-19 Infections: A Prospective Randomized Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.21608/crossmark_policy",
"volume": "7"
}