N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study
et al., Infectious Diseases, doi:10.1080/23744235.2021.1945675, Jun 2021
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 42 hospitalized PCR+ COVID-19 pneumonia patients treated with NAC, and a matched control group of 40 patients, showing significantly lower severe respiratory failure and significantly lower mortality with treatment. NAC 600 mg bid orally for 14 days.
|
risk of death, 97.1% lower, RR 0.03, p = 0.006, treatment 2 of 42 (4.8%), control 12 of 40 (30.0%), NNT 4.0, inverted to make RR<1 favor treatment, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Assimakopoulos et al., 29 Jun 2021, retrospective, Greece, peer-reviewed, 9 authors, study period 1 February, 2021 - 30 April, 2021.
N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study
Infectious Diseases, doi:10.1080/23744235.2021.1945675
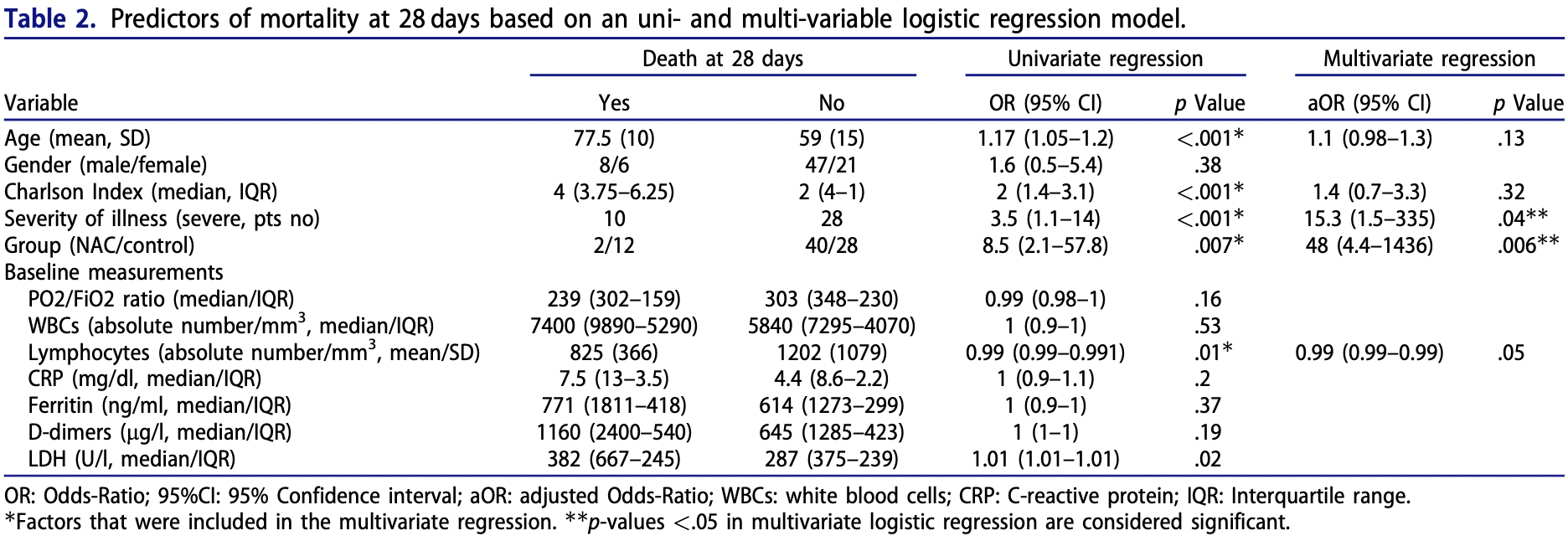
Background: N-acetyl-cysteine (NAC) has been previously shown to exert beneficial effects in diverse respiratory diseases, through antioxidant and anti-inflammatory actions. Our aim was to evaluate NAC potential impact in hospitalised patients with COVID-19 pneumonia, in terms of progression to severe respiratory failure (SRF) and mortality. Patients and Methods: This retrospective, two-centre cohort study included consecutive patients hospitalised with moderate or severe COVID-19 pneumonia. Patients who received standard of care were compared with patients who additionally received NAC 600 mg bid orally for 14 days. Patients' clinical course was recorded regarding (i) the development of SRF (PO 2 /FiO 2 <150) requiring mechanical ventilation support and (ii) mortality at 14 and 28 days. Results: A total of 82 patients were included, 42 in the NAC group and 40 in the control group. Treatment with oral NAC led to significantly lower rates of progression to SRF as compared to the control group (p < .01). Patients in the NAC group presented significantly lower 14-and 28-day mortality as compared to controls (p < .001 and p < .01 respectively). NAC treatment significantly reduced 14-and 28-day mortality in patients with severe disease (p < .001, respectively). NAC improved over time the PO2/FiO2 ratio and decreased the white blood cell, CRP, D-dimers and LDH levels. In the multivariable logistic regression analysis, non-severe illness and NAC administration were independent predictors of 28days survival. Conclusion: Oral NAC administration (1200 mg/d) in patients with COVID-19 pneumonia reduces the risk for mechanical ventilation and mortality. Our findings need to be confirmed by properly designed prospective clinical trials.
Disclosure statement The authors declare that they have no conflict of interest.
ORCID Stelios F. Assimakopoulos http://orcid.org/0000-0002-6901-3681
References
Assimakopoulos, Maroulis, Patsoukis, Effect of antioxidant treatments on the gut-liver axis oxidative status and function in bile duct-ligated rats, World J Surg
Ca, Interpreting indicators of iron status during an acute phase response-lessons from malaria and human immunodeficiency virus, Ann Clin Biochem
Conner, Grisham, Inflammation, free radicals, and antioxidants, Nutrition
De Alencar, Moreira, Muller, Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by COVID-19, Clin Infect Dis
De Souza, Ns, Batista, On the analysis of mortality risk factors for hospitalized COVID-19 patients: A data-driven study using the major Brazilian database, PLoS One
Harvey, Joubert, Preez, Effect of chronic N-acetyl cysteine administration on oxidative status in the presence and absence of induced oxidative stress in rat striatum, Neurochem Res
Horowitz, Freeman, Bruzzese, Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: A report of 2 cases, Respir Med Case Rep
Ibrahim, Smith, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol
Lee, Kang, N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction, Sci Rep
Liu, Yao, Xu, The anti-inflammatory effects of acetaminophen and N-acetylcysteine through suppression of the NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes, Innate Immun
Mata, Morcillo, Gimeno, N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV), Biochem Pharmacol
Mehta, Mcauley, Brown, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Millea, N-acetylcysteine: multiple clinical applications, Am Fam Physician
Sagrista, Garcia, De Madariaga, Antioxidant and pro-oxidant effect of the thiolic compounds N-acetyl-L-cysteine and glutathione against free radical-induced lipid peroxidation, Free Radic Res
Sanguinetti, N-acetylcysteine in COPD: why, how, and when? Multidiscip, Respir Med
Scheffel, Scurti, Wyatt, N-acetyl cysteine protects anti-melanoma cytotoxic T cells from exhaustion induced by rapid expansion via the downmodulation of Foxo1 in an Akt-dependent manner, Cancer Immunol Immunother
Selcuk, Cinar, Keskin, Is the use of ACE inb/ ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients?, Clin Exp Hypertens
Sharafkhah, Abdolrazaghnejad, Zarinfar, Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial, Med Gas Res
Shi, Puyo, N-acetylcysteine to combat COVID-19: an evidence review, Ther Clin Risk Manag
Sirivongrangson, Kulvichit, Payungporn, Endotoxemia and circulating bacteriome in severe COVID-19 patients, Intensive Care Med Exp
Sotler, Poljsak, Dahmane, Prooxidant Activities of Antioxidants and Their Impact on Health, Acta Clin Croat
Takahashi, Yoshida, Ohnuma, The enhancing effect of the antioxidant N-acetylcysteine on urinary bladder injury induced by dimethylarsinic acid, Toxicol Pathol
Wang, Wang, Identification of risk factors for in-hospital death of COVID-19 pneumonia -lessions from the early outbreak, BMC Infect Dis
Zhang, Ding, Li, Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: A metaanalysis, Exp Ther Med
Zhang, Ju, Ma, N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial, Medicine
DOI record:
{
"DOI": "10.1080/23744235.2021.1945675",
"ISSN": [
"2374-4235",
"2374-4243"
],
"URL": "http://dx.doi.org/10.1080/23744235.2021.1945675",
"alternative-id": [
"10.1080/23744235.2021.1945675"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=infd20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=infd20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-05-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2021-06-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2021-06-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2021-06-29"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6901-3681",
"affiliation": [
{
"name": "Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"authenticated-orcid": false,
"family": "Assimakopoulos",
"given": "Stelios F.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology and Intensive Care Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Aretha",
"given": "Diamanto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, “St Andrews” State General Hospital, Patras, Greece"
}
],
"family": "Komninos",
"given": "Dimitris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, “St Andrews” State General Hospital, Patras, Greece"
}
],
"family": "Dimitropoulou",
"given": "Dimitra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Lagadinou",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Leonidou",
"given": "Lydia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Oikonomou",
"given": "Ioanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology, Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Mouzaki",
"given": "Athanasia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of Patras Medical School, Patras, Greece"
}
],
"family": "Marangos",
"given": "Markos",
"sequence": "additional"
}
],
"container-title": [
"Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2021,
6,
29
]
],
"date-time": "2021-06-29T08:53:18Z",
"timestamp": 1624956798000
},
"deposited": {
"date-parts": [
[
2021,
11,
4
]
],
"date-time": "2021-11-04T09:59:55Z",
"timestamp": 1636019995000
},
"indexed": {
"date-parts": [
[
2021,
12,
28
]
],
"date-time": "2021-12-28T06:47:55Z",
"timestamp": 1640674075629
},
"is-referenced-by-count": 3,
"issn-type": [
{
"type": "print",
"value": "2374-4235"
},
{
"type": "electronic",
"value": "2374-4243"
}
],
"issue": "11",
"issued": {
"date-parts": [
[
2021,
6,
29
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2021,
11,
2
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/23744235.2021.1945675",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "847-854",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2021,
6,
29
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
29
]
]
},
"published-print": {
"date-parts": [
[
2021,
11,
2
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1016/S0899-9007(96)00000-8",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"key": "CIT0003",
"unstructured": "COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/"
},
{
"DOI": "10.1097/MD.0000000000013087",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.1186/s40248-016-0039-2",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"author": "Millea PJ.",
"first-page": "265",
"issue": "3",
"journal-title": "Am Fam Physician",
"key": "CIT0006",
"volume": "80",
"year": "2009"
},
{
"DOI": "10.1080/10641963.2020.1783549",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1186/s12879-021-05814-4",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1371/journal.pone.0248580",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.1258/acb.2007.007167",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.4103/2045-9912.229599",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.1177/1753425914566205",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1038/s41598-018-37296-x",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"DOI": "10.1007/s00268-007-9191-3",
"doi-asserted-by": "publisher",
"key": "CIT0014"
},
{
"DOI": "10.1186/s40635-020-00362-8",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.1007/s00262-018-2120-5",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1016/j.bcp.2011.05.014",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.2147/TCRM.S273700",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"DOI": "10.1093/cid/ciaa1443",
"doi-asserted-by": "publisher",
"key": "CIT0019"
},
{
"DOI": "10.1016/j.rmcr.2020.101063",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.3892/etm.2017.4891",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"author": "Sotler R",
"first-page": "726",
"issue": "4",
"journal-title": "Acta Clin Croat",
"key": "CIT0023",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.1080/10715760290019354",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1007/s11064-007-9466-y",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"DOI": "10.1177/0192623311422076",
"doi-asserted-by": "publisher",
"key": "CIT0026"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"score": 1,
"short-container-title": [
"Infectious Diseases"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Immunology and Microbiology",
"General Medicine"
],
"subtitle": [],
"title": [
"N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "53"
}
