Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study
et al., ERJ Open Research, doi:10.1183/23120541.00542-2021, STORM, NCT04424992, Dec 2021
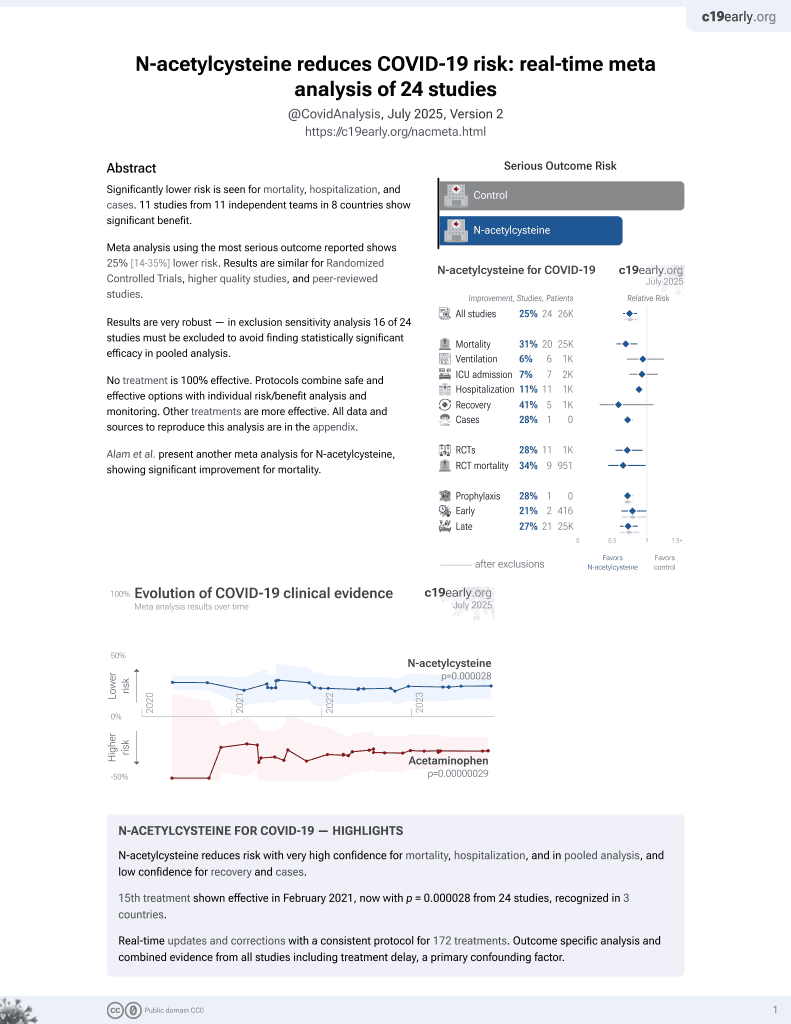
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
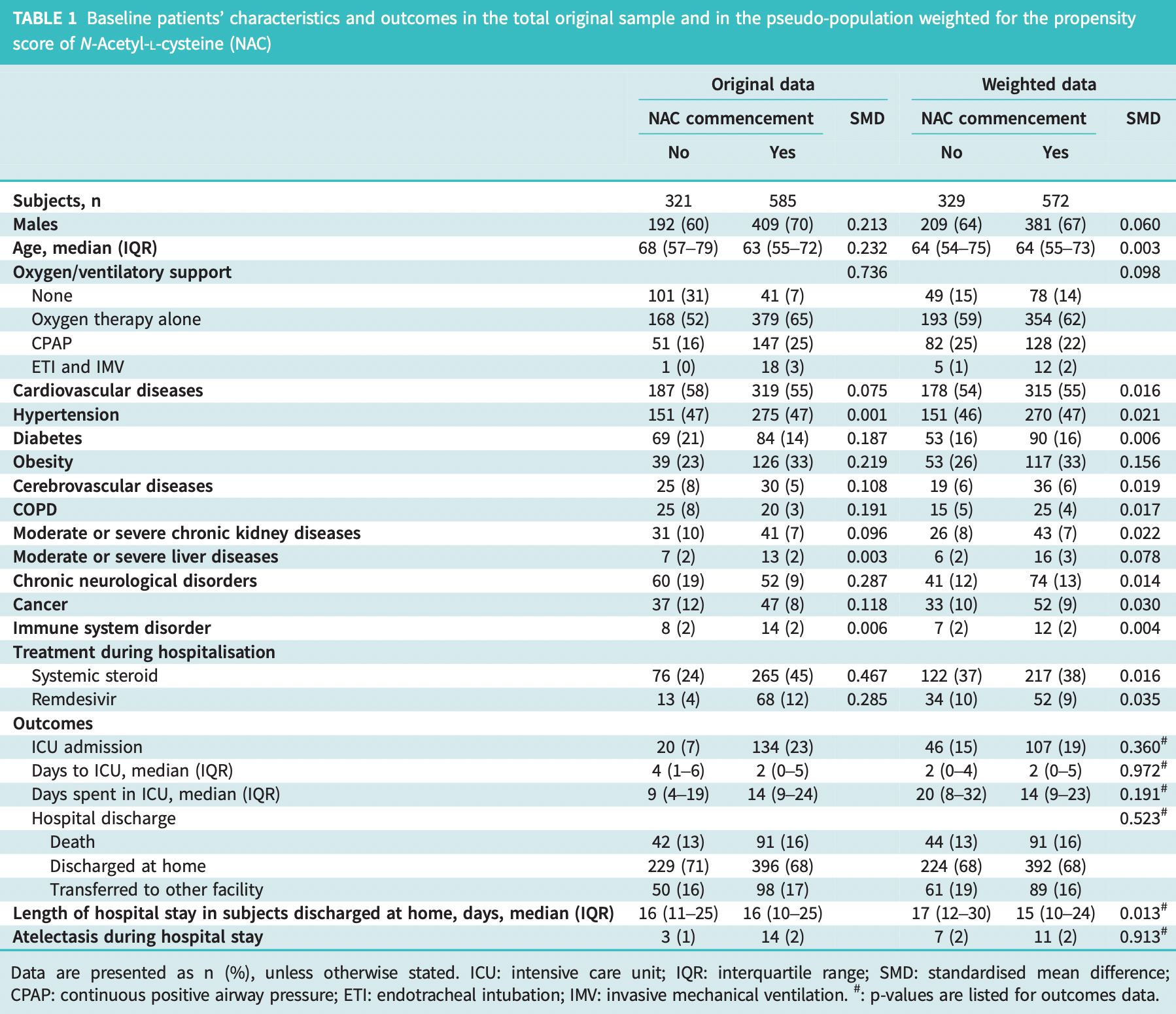
Retrospective 1,083 consecutive hospitalized COVID patients in Italy, showing no significant differences with NAC treatment. The number of patients transferred to another facility exceeds the number of deaths, which may significantly affect results.
|
risk of death, 19.0% higher, RR 1.19, p = 0.33, treatment 91 of 572 (15.9%), control 44 of 329 (13.4%), propensity score weighting.
|
|
risk of ICU admission, 33.8% higher, RR 1.34, p = 0.08, treatment 107 of 572 (18.7%), control 46 of 329 (14.0%), propensity score weighting.
|
|
risk of no hospital discharge, 1.4% lower, RR 0.99, p = 0.94, treatment 180 of 572 (31.5%), control 105 of 329 (31.9%), NNT 224, propensity score weighting.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Faverio et al., 2 Dec 2021, retrospective, Italy, peer-reviewed, 10 authors, study period February 2020 - April 2021, trial NCT04424992 (history) (STORM).
Impact of N-acetyl-L-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study
doi:10.1183/23120541.00542-2021].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may cause pneumonia and acute respiratory distress syndrome (ARDS), whose pathogenesis has been partially related to an increased systemic inflammatory response with great production of pro-inflammatory cytokines causing a "cytokine storm" and an oxidative stress imbalance [1]. N-acetyl-L-cysteine (NAC) is a precursor of reduced glutathione [2] that has antioxidant, anti-inflammatory and immunomodulating properties that may prove beneficial in modulating the excessive inflammatory activation during coronavirus disease (COVID-19) [3]. Furthermore, NAC has been extensively used as a mucolytic agent to improve airway clearance in chronic respiratory diseases. During the COVID-19 pandemic research hypotheses on the role of NAC have been formulated and randomised control trials (RCT) are ongoing; however, to date only a few case reports have been published [3, 4]. The aim of our study is to evaluate the impact of NAC administered during hospitalisation for SARS-CoV-2 pneumonia on short-term and long-term outcomes. As short-term outcomes we considered in-hospital mortality, intensive care unit (ICU) admission, length of ICU stay and length of hospital stay (LOS) in patients discharged alive; as long-term outcomes we included diffusion capacity of the lung for carbon monoxide (D LCO ) impairment, chest radiography alterations, reduced distance walked in the 6-min walk test (6MWT) and dyspnoea score (modified Medical Research Council (mMRC) scale) at 6 months follow-up in a subset of patients included in a follow-up study. Furthermore, we will also evaluate the impact of NAC on the development of atelectasis during hospitalisation, a possible complication of SARS-CoV-2 pneumonia. We performed a retrospective monocentric study on 1083 consecutive adult patients hospitalised for SARS-CoV-2 pneumonia at the San Gerardo Hospital, Monza, Italy, between February 2020 and April 2021. Given that the aim was to evaluate the impact of a least 5 days of NAC administration, patients were excluded if they died or were discharged within 5 days from admission (n=177) to avoid immortal time bias. NAC was introduced, as per institutional protocol, on admission and administered at a dosage of 300 mg intravenously three times daily, switched to 600 mg per os twice daily once the patient reached clinical stability and continued until discharge. The study (STORM) was approved by the national Institutional Review Board (Spallanzani Hospital), and registered at ClinicalTrials.gov with identifier NCT04424992. As part of a multi-centre prospective study to evaluate pulmonary sequelae caused by SARS-CoV-2 pneumonia [5] (ClinicalTrials.gov identifier: NCT04435327), we also had available follow-up data on 102 patients from the original cohort alive at discharge. The follow-up consisted of a pneumological visit at 6 months including complete pulmonary function tests and D LCO , 6MWT, mMRC scale and..
Author contributions: A. Pesci and M.G. Valsecchi are the guarantors of this research. P. Faverio, P. Rebora, E. Rossi, S. Busnelli and A. Pesci were responsible for study concept and design. P. Faverio, S. del Giudice, F. Montanelli, L. Garzillo, S. Busnelli and F. Luppi contributed to patient recruitment and follow-up. All authors contributed to data acquisition. P. Faverio, P. Rebora, E. Rossi, S. Busnelli and M.G. Valsecchi performed data analysis. P. Faverio, P. Rebora, S. Busnelli and F. Luppi contributed to the drafting of this manuscript. All authors read and approved the final manuscript.
DOI record:
{
"DOI": "10.1183/23120541.00542-2021",
"ISSN": [
"2312-0541"
],
"URL": "http://dx.doi.org/10.1183/23120541.00542-2021",
"alternative-id": [
"10.1183/23120541.00542-2021"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0360-1237",
"affiliation": [],
"authenticated-orcid": false,
"family": "Faverio",
"given": "Paola",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rebora",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossi",
"given": "Emanuela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Giudice",
"given": "Savino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montanelli",
"given": "Filippo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garzillo",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Busnelli",
"given": "Sara",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5775-5947",
"affiliation": [],
"authenticated-orcid": false,
"family": "Luppi",
"given": "Fabrizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valsecchi",
"given": "Maria Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pesci",
"given": "Alberto",
"sequence": "additional"
}
],
"container-title": [
"ERJ Open Research"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ersjournals.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
2
]
],
"date-time": "2021-12-02T13:03:10Z",
"timestamp": 1638450190000
},
"deposited": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T10:11:14Z",
"timestamp": 1644228674000
},
"indexed": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T10:41:57Z",
"timestamp": 1644230517880
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2312-0541"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12,
2
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
2,
7
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
2
]
],
"date-time": "2021-12-02T00:00:00Z",
"timestamp": 1638403200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/23120541.00542-2021",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "00542-2021",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2021,
12,
2
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"DOI": "10.1002/jmv.26232",
"article-title": "The cytokine storm and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "250",
"journal-title": "J Med Virol",
"key": "2022020702100780000_8.1.00542-2021.1",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1096/fj.202001807",
"article-title": "Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19, 2020",
"author": "De Flora",
"doi-asserted-by": "crossref",
"first-page": "13185",
"journal-title": "FASEB J",
"key": "2022020702100780000_8.1.00542-2021.2",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.2147/TCRM.S273700",
"article-title": "N-acetylcysteine to combat COVID-19: an evidence review. Therapeutics and clinical risk management",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "1047",
"journal-title": "Ther Clin Risk Manag",
"key": "2022020702100780000_8.1.00542-2021.3",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"doi-asserted-by": "publisher",
"key": "2022020702100780000_8.1.00542-2021.4"
},
{
"DOI": "10.1159/000518141",
"article-title": "Six-month pulmonary impairment after severe COVID-19: a prospective, multicenter follow-up study",
"author": "Faverio",
"doi-asserted-by": "crossref",
"first-page": "1078",
"journal-title": "Respiration",
"key": "2022020702100780000_8.1.00542-2021.5",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1093/biomet/ast027",
"doi-asserted-by": "publisher",
"key": "2022020702100780000_8.1.00542-2021.6"
},
{
"DOI": "10.1093/cid/ciaa1443",
"article-title": "Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by Coronavirus Disease 2019 (COVID-19), 2021",
"author": "de Alencar",
"doi-asserted-by": "crossref",
"first-page": "e736",
"journal-title": "Clin Infect Dis",
"key": "2022020702100780000_8.1.00542-2021.7",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1007/s43440-021-00296-2",
"article-title": "A pilot study on intravenous N-acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome",
"author": "Taher",
"doi-asserted-by": "crossref",
"first-page": "1650",
"journal-title": "Pharmacol Rep",
"key": "2022020702100780000_8.1.00542-2021.8",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2022020702100780000_8.1.00542-2021.9"
},
{
"DOI": "10.1007/s11606-020-06146-w",
"article-title": "Chloroquine and hydroxychloroquine for the treatment of COVID-19: a systematic review and meta-analysis, 2020",
"author": "Elavarasi",
"doi-asserted-by": "crossref",
"first-page": "3308",
"journal-title": "J Gen Intern Med",
"key": "2022020702100780000_8.1.00542-2021.10",
"volume": "35",
"year": "2020"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"score": 1,
"short-container-title": [
"ERJ Open Res"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": [
"Impact of N-acetyl-l-cysteine on SARS-CoV-2 pneumonia and its sequelae: results from a large cohort study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1183/ers-crossmark-policy",
"volume": "8"
}