Evaluation of the efficacy and safety of oral N‐acetylcysteine in patients with COVID‐19 receiving the routine antiviral and hydroxychloroquine protocol: A randomized controlled clinical trial
et al., Immunity, Inflammation and Disease, doi:10.1002/iid3.1083, IRCT20200623047897N1, Nov 2023
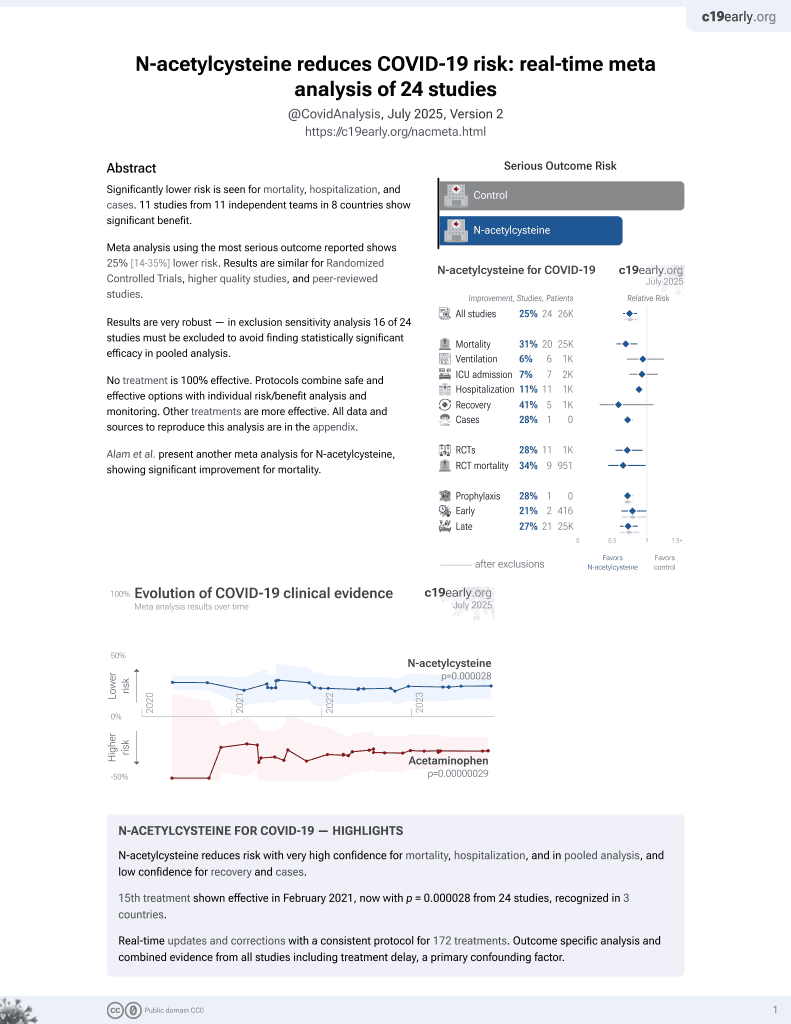
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 60 hospitalized COVID-19 patients evaluating the efficacy and safety of adding oral N-acetylcysteine (NAC) at 600mg three times daily to standard antiviral treatment regimens. The NAC group showed significantly greater reduction in C-reactive protein levels, indicating reduced inflammation. Authors conclude that oral NAC may provide benefits through reducing inflammation, increasing oxygen saturation, and potentially reducing mortality when combined with certain antiviral medications in hospitalized COVID-19 patients.
|
risk of death, 66.7% lower, RR 0.33, p = 0.61, treatment 1 of 30 (3.3%), control 3 of 30 (10.0%), NNT 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Atefi et al., 20 Nov 2023, Single Blind Randomized Controlled Trial, Iran, peer-reviewed, 10 authors, trial IRCT20200623047897N1.
Contact: goodarzi.a@iums.ac.ir, azadeh_goodarzi1984@yahoo.com.
Evaluation of the efficacy and safety of oral N‐acetylcysteine in patients with COVID‐19 receiving the routine antiviral and hydroxychloroquine protocol: A randomized controlled clinical trial
Immunity, Inflammation and Disease, doi:10.1002/iid3.1083
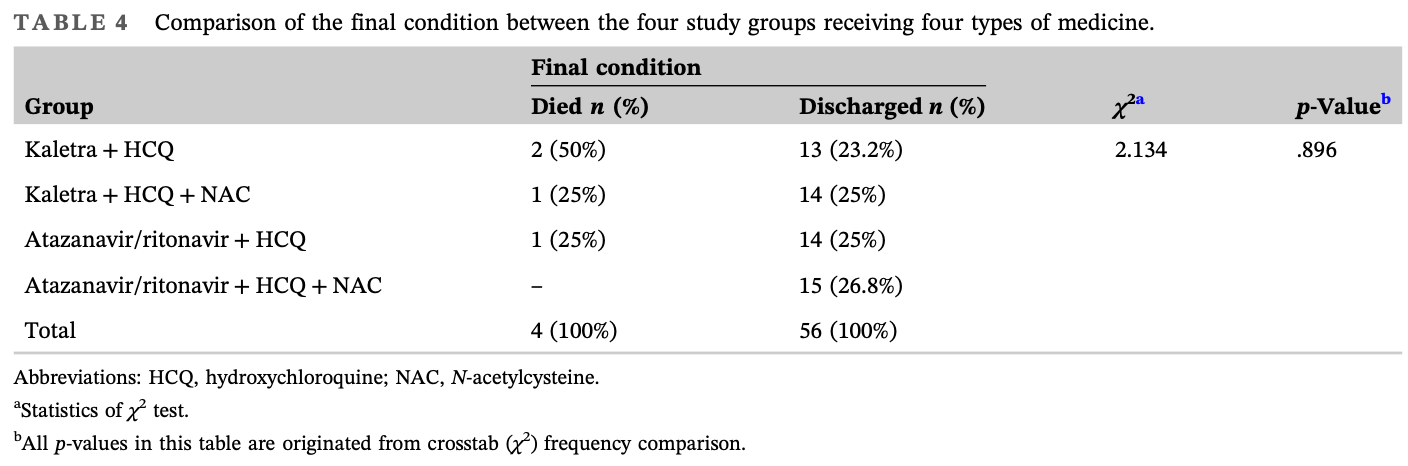
Background: The current absence of gold-standard or all-aspect favorable therapies for COVID-19 renders a focus on multipotential drugs proposed to prevent or treat this infection or ameliorate its signs and symptoms vitally important. The present well-designed randomized controlled trial (RCT) sought to evaluate the efficacy and safety of N-acetylcysteine (NAC) as adjuvant therapy for 60 hospitalized Iranian patients with COVID-19. Methods: Two 30-person diets, comprising 15 single diets of Kaletra (lopinavir/ritonavir) + hydroxychloroquine (HCQ) with/without NAC (600 mg TDS) and atazanavir/ritonavir + HCQ with/without NAC (600 mg TDS), were administered in the study. Results: At the end of the study, a further decrease in C-reactive protein was observed in the NAC group (P = 0.008), and no death occurred in the atazanavir/ritonavir + HCQ + NAC group, showing that the combination of these drugs may reduce mortality. The atazanavir/ritonavir + HCQ and atazanavir/ritonavir + NAC groups exhibited the highest O 2 saturation at the end of the study and a significant rise in O 2 saturation following intervention commencement, including NAC (P > 0.05). Accordingly, oral or intravenous NAC, if indicated, may enhance O 2 saturation, blunt the inflammation trend (by reducing C-reactive protein), and lower mortality in hospitalized patients with COVID-19.
AUTHOR CONTRIBUTIONS Azadeh Goodarzi and Najmolsadat Atefi conceived and planned the intervention. Taghi Riahi, Niloofar Khodabandehloo, Mahshid Talebi Taher and Niloufar Najar Nobari carried out the intervention. Zeinab Mahdi and Amir Baghestani gathering the datas. Amir Baghestani, Zeinab Mahdi, Rohollah Valizadeh, and Farnoosh Seirafianpour contributed to the interpretation of the results. Rohollah Valizadeh analyzed the data. Farnoosh Seirafianpour took the lead in writing the manuscript. All authors contributed to the preparation of data and the finalization of this article. All the figures have been produced by the authors of this article and are personal data.
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
References
Ahmed, Chakrabarty, Guengerich, Chowdhury, Protective role of glutathione against peroxynitrite-mediated DNA damage during acute inflammation, Chem Res Toxicol
Alamdari, Moghaddam, Amini, Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial, Eur J Pharmacol
Andreou, Trantza, Filippou, Sipsas, Tsiodras, COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2, Vivo
Aslan, Aslan, Zolbanin, Jafari, Acute respiratory distress syndrome in COVID-19: possible mechanisms and therapeutic management, Pneumonia, doi:10.1186/s41479-021-00092-9
Assimakopoulos, Marangos, N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome, Med Hypotheses
Baud, Qi, Nielsen-Saines, Musso, Pomar et al., Real estimates of mortality following COVID-19 infection, Lancet Infect Dis
Bhat, Singh, Bhat, Borole, Khan, Coronavirus disease-2019 and its current scenario-a review, Clinical eHealth, doi:10.1016/j.ceh.2021.09.002
Bourgonje, Offringa, Van Eijk, N-acetylcysteine and hydrogen sulfide in coronavirus disease 2019, Antioxid Redox Signal
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord
Carothers, Birrer, Vo, Acetylcysteine for the treatment of suspected remdesivir-associated acute liver failure in COVID-19: a case series, Pharmacotherapy
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet
Daid, Toribio, Lakshmanan, Sadda, Epstein, Spontaneous intraparenchymal hepatic hemorrhage as a sequela of COVID-19, Cureus
Daneshfar, Dadashzadeh, Ahmadpour, Lessons of mortality following COVID-19 epidemic in the United States especially in the geriatrics, J Nephropharmacol
Dass, Brief review of N-acetylcysteine as antiviral agent: potential application in COVID-19, J Biomed Pharm Res
De Alencar, Moreira, Müller, Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID-19), Clin Infect Dis
El-Serafi, Remberger, El-Serafi, The effect of Nacetyl-L-cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation, Sci Rep, doi:10.1038/s41598-018-26033-z
Elgamasy, Kamel, Ghozy, Khalil, Morra et al., First case of focal epilepsy associated with SARScoronavirus-2, J Med Virol
Fang, Karakiulakis, Roth, Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?, Respir Med
Flora, Balansky, Maestra, Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19, FASEB J
Fregatti, Gipponi, Giacchino, Breast cancer surgery in the COVID-19 pandemic: validation of a preventive program for patients and health care workers, Vivo
Goodarzi, A comprehensive review on COVID-19 infection and comorbidities of various organs, Acta Med Iranica
Goodnough, Canseco, Response to comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID-19 pandemic, Clin Toxicol
Goodnough, Canseco, Truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID-19 pandemic, Clin Toxicol
Hamad, MNa theory: triple therapy to COVID-19: minocycline, N-acetylcysteine and aspirin, Saudi J Biomed Res
Hasan, N-acetylcysteine in severe COVID-19: the possible mechanism, Int J Infect
Hatami, Kalani, Javdani, N-acetyl cysteine (NAC) and COVID-19 treatment: new hopes in old medication, Int J Multidiscip Res Anal
Horowitz, Freeman, Bruzzese, Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases, Respir Med Case Rep
Horowitz, Freeman, Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials, Med Hypotheses
Hołyńska ; Iwan, Wróblewski, Olszewska-Słonina, Tyrakowski, The application of N-acetylcysteine in optimization of specific pharmacological therapies, Pol Merkur Lekarski
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol
Ibrahim, Smith, Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin Immunol
Jaiswal, Bhatnagar, Shah, N-acetycysteine: a potential therapeutic agent in COVID-19 infection, Med Hypotheses
Jayaweera, Perera, Gunawardana, Manatunge, Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy, Environ Res, doi:10.1016/j.envres.2020.109819
Kalantari, Sadeghzadeh-Bazargan, Ebrahimi, The effect of influenza vaccine on severity of COVID-19 infection: an original study from Iran, Med J Islam Repub Iran
Kooranifar, Sadeghipour, Riahi, Goodarzi, Tabrizi et al., Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID-19: a large study from Iran with a high rate of anthracosis, Med J Islam Repub Iran
Krynytska, Marushchak, Birchenko, Dovgalyuk, Tokarskyy, COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review), Iran J Microbiol, doi:10.18502/ijm.v13i6.8072
Laforge, Elbim, Frère, Tissue damage from neutrophil-induced oxidative stress in COVID-19, Nat Rev Immunol
Long, Carius, Chavez, Clinical update on COVID-19 for the emergency clinician: presentation and evaluation, Am J Emerg Med, doi:10.1016/j.ajem.2022.01.028
Luo, Luo, Liu, Liu, Li, Perspectives for the use of Nacetylcysteine as a candidate drug to treat COVID-19, Mini Rev Med Chem
Mashayekhi, Seirafianpour, Mohammad, Goodarzi, Severe and life-threatening COVID-19-related mucocutaneous eruptions: a systematic review, Int J Clin Pract
Meletis, Wilkes, Immune competence and minimizing susceptibility to COVID-19 and other immune system threats, Altern Ther Health Med
Mohamadi, Goodarzi, Aryannejad, Geriatric challenges in the new coronavirus disease-19 (COVID-19) pandemic: a systematic review, Med J Islam Repub Iran
Mohanty, Padhy, Das, Meher, Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: review of current evidence, Eur Rev Med Pharmacol Sci
Najafabadi, Rayner, Shokraee, Obesity as an independent risk factor for COVID-19 severity and mortality, Cochrane Database Syst Rev, doi:10.1002/14651858.CD015201
Nasi, Mcardle, Gaudernack, Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider Nacetylcysteine as early therapeutic intervention, Toxicol Rep
Nobari, Montazer, Seirafianpour, Goodarzi, Histopathologic changes and cellular events of organs systems in COVID-19, J Cell Mol Anesth
Pfortmueller, Spinetti, Urman, Luedi, Schefold, COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment-a narrative review, Best Pract Res Clin Anaesthesiol, doi:10.1016/j.bpa.2020.12.011
Poe, Corn, N-acetylcysteine: a potential therapeutic agent for SARS-CoV-2, Med Hypotheses
Riahi, Sadeghzadeh-Bazargan, Shokri, Evaluation of the efficacy and safety of oral N-acetylcysteine in patients with COVID-19 receiving the routine antiviral and hydroxychloroquine protocol: a randomized controlled clinical trial, Med J Islam Repub Iran
Rojas, COVID-19 neurologic manifestation, Rev Fac Cien Med Univ Nac Cordoba
Sadeghzadeh-Bazargan, Behrangi, Goodarzi, Cytokine storm and probable role of immunoregulatory drugs in COVID-19: a comprehensive review, Iranian J Dermatol
Sadeghzadeh-Bazargan, Behrangi, Goodarzi, Systemic retinoids in the COVID-19 era-are they helpful, safe, or harmful? A comprehensive systematized review, Iran J Dermatol
Sarkar, Rapista, Jean, Corona virus disease-19-induced acute liver failure leading to severe metabolic acidosis, Chest
Seirafianpour, Mozafarpoor, Fattahi, Sadeghzadeh-Bazargan, Hanifiha et al., Treatment of COVID-19 with pentoxifylline: could it be a potential adjuvant therapy?, Dermatol Ther
Seirafianpour, Sodagar, Mohammad, Cutaneous manifestations and considerations in COVID-19 pandemic: a systematic review, Dermatol Ther
Shi, Puyo, N-acetylcysteine to combat COVID-19: an evidence review, Ther Clin Risk Manag
Shokraee, Mahdavi, Panahi, Accuracy of chest computed tomography and reverse transcription polymerase chain reaction in diagnosis of 2019 novel coronavirus disease; a systematic review and meta-analysis, Immunopathol Persa
Tavakolpour, Aryanian, Seirafianpour, A systematic review on efficacy, safety, and treatmentdurability of low-dose rituximab for the treatment of pemphigus: special focus on COVID-19 pandemic concerns, Immunopharmacol Immunotoxicol
Van Hecke, Lee, N-acetylcysteine: a rapid review of the evidence for effectiveness in treating COVID-19
Wong, Comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID-19 pandemic, Clin Toxicol
Yüce, Filiztekin, Özkaya, COVID-19 diagnosis-a review of current methods, Biosens Bioelectron, doi:10.1016/j.bios.2020.112752
Zhou, Yang, Huang, Chen, The potential mechanism of N-acetylcysteine in treating COVID-19, Curr Pharm Biotechnol
DOI record:
{
"DOI": "10.1002/iid3.1083",
"ISSN": [
"2050-4527",
"2050-4527"
],
"URL": "http://dx.doi.org/10.1002/iid3.1083",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>The current absence of gold‐standard or all‐aspect favorable therapies for COVID‐19 renders a focus on multipotential drugs proposed to prevent or treat this infection or ameliorate its signs and symptoms vitally important. The present well‐designed randomized controlled trial (RCT) sought to evaluate the efficacy and safety of N‐acetylcysteine (NAC) as adjuvant therapy for 60 hospitalized Iranian patients with COVID‐19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Two 30‐person diets, comprising 15 single diets of Kaletra (lopinavir/ritonavir) + hydroxychloroquine (HCQ) with/without NAC (600 mg TDS) and atazanavir/ritonavir + HCQ with/without NAC (600 mg TDS), were administered in the study.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>At the end of the study, a further decrease in C‐reactive protein was observed in the NAC group (<jats:italic>P</jats:italic> = 0.008), and no death occurred in the atazanavir/ritonavir + HCQ + NAC group, showing that the combination of these drugs may reduce mortality. The atazanavir/ritonavir + HCQ and atazanavir/ritonavir + NAC groups exhibited the highest O<jats:sub>2</jats:sub> saturation at the end of the study and a significant rise in O<jats:sub>2</jats:sub> saturation following intervention commencement, including NAC (<jats:italic>P</jats:italic> > 0.05). Accordingly, oral or intravenous NAC, if indicated, may enhance O<jats:sub>2</jats:sub> saturation, blunt the inflammation trend (by reducing C‐reactive protein), and lower mortality in hospitalized patients with COVID‐19.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The NAC could be more effective as prophylactic or adjuvant therapy in stable non‐severe cases of COVID‐19 with a particularly positive role in the augmentation of O<jats:sub>2</jats:sub> saturation and faster reduction of the CRP level and inflammation or could be effective for better controlling of COVID‐19 or its therapy‐related side effects.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/iid3.1083"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-06-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-10-31"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-11-20"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1053-2441",
"affiliation": [
{
"name": "Department of Dermatology, Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Atefi",
"given": "Najmolsadat",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1249-4429",
"affiliation": [
{
"name": "Department of Dermatology, Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Goodarzi",
"given": "Azadeh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7381-0790",
"affiliation": [
{
"name": "Department of Internal Medicine, School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Riahi",
"given": "Taghi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0307-6014",
"affiliation": [
{
"name": "Department of Geriatric Medicine, School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Khodabandehloo",
"given": "Niloofar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3133-7859",
"affiliation": [
{
"name": "Department of Infectious Disease, School of Medicine, Antimicrobial Resistance Research Center, Immunology and Infectious Disease Research Institute Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Talebi Taher",
"given": "Mahshid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4245-1980",
"affiliation": [
{
"name": "Department of Dermatology, Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Najar Nobari",
"given": "Niloufar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3794-6206",
"affiliation": [
{
"name": "Razi Drug Research Center Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Seirafianpour",
"given": "Farnoosh",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1114-8660",
"affiliation": [
{
"name": "Department of Dermatology, Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Mahdi",
"given": "Zeinab",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of General Medicine, Rasool Akram Medical Complex, School of Medicine Iran University of Medical Sciences Tehran Iran"
}
],
"family": "Baghestani",
"given": "Amir",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6913-3210",
"affiliation": [
{
"name": "Urmia University of Medical Sciences Urmia Iran"
}
],
"authenticated-orcid": false,
"family": "Valizadeh",
"given": "Rohollah",
"sequence": "additional"
}
],
"container-title": "Immunity, Inflammation and Disease",
"container-title-short": "Immunity Inflam &amp; Disease",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T21:42:09Z",
"timestamp": 1700602929000
},
"deposited": {
"date-parts": [
[
2023,
11,
21
]
],
"date-time": "2023-11-21T21:42:42Z",
"timestamp": 1700602962000
},
"indexed": {
"date-parts": [
[
2023,
11,
22
]
],
"date-time": "2023-11-22T00:26:04Z",
"timestamp": 1700612764728
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 19,
"start": {
"date-parts": [
[
2023,
11,
20
]
],
"date-time": "2023-11-20T00:00:00Z",
"timestamp": 1700438400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/iid3.1083",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
20
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "N‐acetylcysteine as a potential treatment for COVID‐19",
"author": "Jorge‐Aarón R‐M",
"first-page": "959",
"journal-title": "Future Med",
"key": "e_1_2_10_2_1",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"article-title": "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster",
"author": "Chan JFW",
"doi-asserted-by": "crossref",
"first-page": "514",
"issue": "10223",
"journal-title": "Lancet",
"key": "e_1_2_10_3_1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.envres.2020.109819",
"article-title": "Transmission of COVID‐19 virus by droplets and aerosols: a critical review on the unresolved dichotomy",
"author": "Jayaweera M",
"doi-asserted-by": "crossref",
"journal-title": "Environ Res",
"key": "e_1_2_10_4_1",
"volume": "188",
"year": "2020"
},
{
"article-title": "Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection?",
"author": "Fang L",
"issue": "4",
"journal-title": "Respir Med",
"key": "e_1_2_10_5_1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.ceh.2021.09.002",
"article-title": "Coronavirus disease‐2019 and its current scenario—a review",
"author": "Bhat SA",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "Clinical eHealth",
"key": "e_1_2_10_6_1",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1111/dth.13986",
"article-title": "Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review",
"author": "Seirafianpour F",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Dermatol Ther",
"key": "e_1_2_10_7_1",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1186/s41479-021-00092-9",
"article-title": "Acute respiratory distress syndrome in COVID‐19: possible mechanisms and therapeutic management",
"author": "Aslan A",
"doi-asserted-by": "crossref",
"first-page": "14",
"issue": "1",
"journal-title": "Pneumonia",
"key": "e_1_2_10_8_1",
"volume": "13",
"year": "2021"
},
{
"article-title": "COVID‐19‐associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review)",
"author": "Krynytska I",
"first-page": "737",
"issue": "6",
"journal-title": "Iran J Microbiol",
"key": "e_1_2_10_9_1",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.bpa.2020.12.011",
"article-title": "COVID‐19‐associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment—a narrative review",
"author": "Pfortmueller CA",
"doi-asserted-by": "crossref",
"first-page": "351",
"issue": "3",
"journal-title": "Best Pract Res Clin Anaesthesiol",
"key": "e_1_2_10_10_1",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.ajem.2022.01.028",
"article-title": "Clinical update on COVID‐19 for the emergency clinician: presentation and evaluation",
"author": "Long B",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Am J Emerg Med",
"key": "e_1_2_10_11_1",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26232",
"article-title": "The cytokine storm and COVID‐19",
"author": "Hu B",
"doi-asserted-by": "crossref",
"first-page": "250",
"issue": "1",
"journal-title": "J Med Virol",
"key": "e_1_2_10_12_1",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30195-X",
"article-title": "Real estimates of mortality following COVID‐19 infection",
"author": "Baud D",
"doi-asserted-by": "crossref",
"first-page": "773",
"issue": "7",
"journal-title": "Lancet Infect Dis",
"key": "e_1_2_10_13_1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.34172/npj.2021.06",
"article-title": "Lessons of mortality following COVID‐19 epidemic in the United States especially in the geriatrics",
"author": "Daneshfar M",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J Nephropharmacol",
"key": "e_1_2_10_14_1",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1080/08923973.2021.1953063",
"article-title": "A systematic review on efficacy, safety, and treatment‐durability of low‐dose rituximab for the treatment of pemphigus: special focus on COVID‐19 pandemic concerns",
"author": "Tavakolpour S",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Immunopharmacol Immunotoxicol",
"key": "e_1_2_10_15_1",
"volume": "43",
"year": "2021"
},
{
"article-title": "Brief review of N‐acetylcysteine as antiviral agent: potential application in COVID‐19",
"author": "Dass E",
"first-page": "69",
"issue": "3",
"journal-title": "J Biomed Pharm Res",
"key": "e_1_2_10_16_1",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.ejphar.2020.173494",
"article-title": "Application of methylene blue‐vitamin C–N‐acetyl cysteine for treatment of critically ill COVID‐19 patients, report of a phase‐I clinical trial",
"author": "Alamdari DH",
"doi-asserted-by": "crossref",
"journal-title": "Eur J Pharmacol",
"key": "e_1_2_10_17_1",
"volume": "885",
"year": "2020"
},
{
"DOI": "10.21873/invivo.11946",
"article-title": "COVID‐19: the potential role of copper and N‐acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS‐CoV‐2",
"author": "Andreou A",
"doi-asserted-by": "crossref",
"first-page": "1567",
"issue": "3",
"journal-title": "In Vivo",
"key": "e_1_2_10_18_1",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.36348/sjbr.2020.v05i04.003",
"article-title": "MNa theory: triple therapy to COVID‐19: minocycline, N‐acetylcysteine and aspirin",
"author": "Hamad MNM",
"doi-asserted-by": "crossref",
"first-page": "53",
"issue": "4",
"journal-title": "Saudi J Biomed Res",
"key": "e_1_2_10_19_1",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.110133",
"article-title": "N‐acetycysteine: a potential therapeutic agent in COVID‐19 infection",
"author": "Jaiswal N",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses",
"key": "e_1_2_10_20_1",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1021/acs.chemrestox.0c00299",
"article-title": "Protective role of glutathione against peroxynitrite‐mediated DNA damage during acute inflammation",
"author": "Ahmed N",
"doi-asserted-by": "crossref",
"first-page": "2668",
"issue": "10",
"journal-title": "Chem Res Toxicol",
"key": "e_1_2_10_21_1",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109778",
"article-title": "N‐acetyl‐cysteine may prevent COVID‐19‐associated cytokine storm and acute respiratory distress syndrome",
"author": "Assimakopoulos SF",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses",
"key": "e_1_2_10_22_1",
"volume": "140",
"year": "2020"
},
{
"DOI": "10.5812/iji.106361",
"article-title": "N‐acetylcysteine in severe COVID‐19: the possible mechanism",
"author": "Hasan MJ",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Int J Infect",
"key": "e_1_2_10_23_1",
"volume": "7",
"year": "2020"
},
{
"article-title": "N‐acetyl cysteine (NAC) and COVID‐19 treatment: new hopes in old medication",
"author": "Hatami N",
"first-page": "122",
"issue": "9",
"journal-title": "Int J Multidiscip Res Anal",
"key": "e_1_2_10_24_1",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.2147/TCRM.S273700",
"article-title": "N‐acetylcysteine to combat COVID‐19: an evidence review",
"author": "Shi Z",
"doi-asserted-by": "crossref",
"first-page": "1047",
"journal-title": "Ther Clin Risk Manag",
"key": "e_1_2_10_25_1",
"volume": "16",
"year": "2020"
},
{
"key": "e_1_2_10_26_1",
"unstructured": "Van HeckeO LeeJ.N‐acetylcysteine: a rapid review of the evidence for effectiveness in treating COVID‐19.2020."
},
{
"DOI": "10.1016/j.mehy.2020.109862",
"article-title": "N‐acetylcysteine: a potential therapeutic agent for SARS‐CoV‐2",
"author": "Poe FL",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses",
"key": "e_1_2_10_27_1",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.31053/1853.0605.v77.n2.28410",
"article-title": "COVID‐19 neurologic manifestation",
"author": "Ocampo Rojas SJ",
"doi-asserted-by": "crossref",
"first-page": "130",
"issue": "2",
"journal-title": "Rev Fac Cien Med Univ Nac Cordoba",
"key": "e_1_2_10_28_1",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1002/phar.2464",
"article-title": "Acetylcysteine for the treatment of suspected remdesivir‐associated acute liver failure in COVID‐19: a case series",
"author": "Carothers C",
"doi-asserted-by": "crossref",
"first-page": "1166",
"issue": "11",
"journal-title": "Pharmacotherapy",
"key": "e_1_2_10_29_1",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.08.932",
"article-title": "Corona virus disease‐19‐induced acute liver failure leading to severe metabolic acidosis",
"author": "Sarkar S",
"doi-asserted-by": "crossref",
"first-page": "A1002",
"issue": "4",
"journal-title": "Chest",
"key": "e_1_2_10_30_1",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1038/s41598-018-26033-z",
"article-title": "The effect of N‐acetyl‐l‐cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation",
"author": "El‐Serafi I",
"doi-asserted-by": "crossref",
"first-page": "8293",
"issue": "1",
"journal-title": "Sci Rep",
"key": "e_1_2_10_31_1",
"volume": "8",
"year": "2018"
},
{
"article-title": "[The application of N‐acetylcysteine in optimization of specific pharmacological therapies]",
"author": "Hołyńska‐Iwan I",
"first-page": "140",
"issue": "255",
"journal-title": "Pol Merkur Lekarski",
"key": "e_1_2_10_32_1",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.1186/s12902-020-00626-0",
"article-title": "Repurposing existing drugs for COVID‐19: an endocrinology perspective",
"author": "Cadegiani FA",
"doi-asserted-by": "crossref",
"first-page": "149",
"issue": "1",
"journal-title": "BMC Endocr Disord",
"key": "e_1_2_10_33_1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1096/fj.202001807",
"article-title": "Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19",
"author": "De Flora S",
"doi-asserted-by": "crossref",
"first-page": "13185",
"issue": "10",
"journal-title": "FASEB J",
"key": "e_1_2_10_34_1",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.21873/invivo.12302",
"article-title": "Breast cancer surgery in the COVID‐19 pandemic: validation of a preventive program for patients and health care workers",
"author": "Fregatti P",
"doi-asserted-by": "crossref",
"first-page": "635",
"issue": "1",
"journal-title": "In Vivo",
"key": "e_1_2_10_35_1",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1443",
"article-title": "Double‐blind, randomized, placebo‐controlled trial with N‐acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID‐19)",
"author": "Alencar JCG",
"doi-asserted-by": "crossref",
"first-page": "e736",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "e_1_2_10_36_1",
"volume": "72",
"year": "2021"
},
{
"article-title": "Spontaneous intraparenchymal hepatic hemorrhage as a sequela of COVID‐19",
"author": "Daid SS",
"issue": "9",
"journal-title": "Cureus",
"key": "e_1_2_10_37_1",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26113",
"article-title": "First case of focal epilepsy associated with SARS‐coronavirus‐2",
"author": "Elgamasy S",
"doi-asserted-by": "crossref",
"first-page": "2238",
"issue": "10",
"journal-title": "J Med Virol",
"key": "e_1_2_10_38_1",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1080/15563650.2020.1758327",
"article-title": "Truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic",
"author": "Goodnough R",
"doi-asserted-by": "crossref",
"first-page": "69",
"issue": "1",
"journal-title": "Clin Toxicol",
"key": "e_1_2_10_39_1",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1080/15563650.2020.1813298",
"article-title": "Response to comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic",
"author": "Goodnough R",
"doi-asserted-by": "crossref",
"first-page": "78",
"issue": "1",
"journal-title": "Clin Toxicol",
"key": "e_1_2_10_40_1",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.109851",
"article-title": "Three novel prevention, diagnostic, and treatment options for COVID‐19 urgently necessitating controlled randomized trials",
"author": "Horowitz RI",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses",
"key": "e_1_2_10_41_1",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108544",
"article-title": "Therapeutic blockade of inflammation in severe COVID‐19 infection with intravenous N‐acetylcysteine",
"author": "Ibrahim H",
"doi-asserted-by": "crossref",
"journal-title": "Clin Immunol",
"key": "e_1_2_10_42_1",
"volume": "219",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0407-1",
"article-title": "Tissue damage from neutrophil‐induced oxidative stress in COVID‐19",
"author": "Laforge M",
"doi-asserted-by": "crossref",
"first-page": "515",
"issue": "9",
"journal-title": "Nat Rev Immunol",
"key": "e_1_2_10_43_1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.2174/1389557520666201027160833",
"article-title": "Perspectives for the use of N‐acetylcysteine as a candidate drug to treat COVID‐19",
"author": "Luo P",
"doi-asserted-by": "crossref",
"first-page": "268",
"issue": "3",
"journal-title": "Mini Rev Med Chem",
"key": "e_1_2_10_44_1",
"volume": "21",
"year": "2021"
},
{
"article-title": "Immune competence and minimizing susceptibility to COVID‐19 and other immune system threats",
"author": "Meletis CD",
"first-page": "94",
"issue": "2",
"journal-title": "Altern Ther Health Med",
"key": "e_1_2_10_45_1",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.toxrep.2020.06.003",
"article-title": "Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS‐CoV‐2 in an ageing population, consider N‐acetylcysteine as early therapeutic intervention",
"author": "Nasi A",
"doi-asserted-by": "crossref",
"first-page": "768",
"journal-title": "Toxicol Rep",
"key": "e_1_2_10_46_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1080/15563650.2020.1809667",
"article-title": "Comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic",
"author": "Wong A",
"doi-asserted-by": "crossref",
"first-page": "77",
"issue": "1",
"journal-title": "Clin Toxicol",
"key": "e_1_2_10_47_1",
"volume": "59",
"year": "2021"
},
{
"DOI": "10.2174/18734316MTEyyNzY6y",
"article-title": "The potential mechanism of N‐acetylcysteine in treating COVID‐19",
"author": "Zhou N",
"doi-asserted-by": "crossref",
"first-page": "1584",
"journal-title": "Curr Pharm Biotechnol",
"key": "e_1_2_10_48_1",
"volume": "2",
"year": "2021"
},
{
"article-title": "Therapeutic potential of N‐acetyl cysteine (NAC) in preventing cytokine storm in COVID‐19: review of current evidence",
"author": "Mohanty RR",
"first-page": "2802",
"issue": "6",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "e_1_2_10_49_1",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1089/ars.2020.8247",
"article-title": "N‐acetylcysteine and hydrogen sulfide in coronavirus disease 2019",
"author": "Bourgonje AR",
"doi-asserted-by": "crossref",
"first-page": "1207",
"issue": "14",
"journal-title": "Antioxid Redox Signal",
"key": "e_1_2_10_50_1",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/ijcp.14720",
"article-title": "Severe and life‐threatening COVID‐19‐related mucocutaneous eruptions: a systematic review",
"author": "Mashayekhi F",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "Int J Clin Pract",
"key": "e_1_2_10_51_1",
"volume": "75",
"year": "2021"
},
{
"article-title": "Efficacy of glutathione therapy in relieving dyspnea associated with COVID‐19 pneumonia: a report of 2 cases",
"author": "Horowitz RI",
"journal-title": "Respir Med Case Rep",
"key": "e_1_2_10_52_1",
"volume": "30",
"year": "2020"
},
{
"article-title": "Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: a systematic review",
"author": "Mohamadi M",
"first-page": "123",
"journal-title": "Med J Islam Repub Iran",
"key": "e_1_2_10_53_1",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1111/dth.13733",
"article-title": "Treatment of COVID‐19 with pentoxifylline: could it be a potential adjuvant therapy?",
"author": "Seirafianpour F",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Dermatol Ther",
"key": "e_1_2_10_54_1",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.34172/ipp.2021.36",
"article-title": "Accuracy of chest computed tomography and reverse transcription polymerase chain reaction in diagnosis of 2019 novel coronavirus disease; a systematic review and meta‐analysis",
"author": "Shokraee K",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Immunopathol Persa",
"key": "e_1_2_10_55_1",
"volume": "7",
"year": "2021"
},
{
"article-title": "Systemic retinoids in the COVID‐19 era–are they helpful, safe, or harmful? A comprehensive systematized review",
"author": "Sadeghzadeh‐Bazargan A",
"first-page": "9",
"issue": "1",
"journal-title": "Iran J Dermatol",
"key": "e_1_2_10_56_1",
"volume": "23",
"year": "2020"
},
{
"article-title": "Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review",
"author": "Sadeghzadeh‐Bazargan A",
"first-page": "13",
"issue": "1",
"journal-title": "Iranian J Dermatol",
"key": "e_1_2_10_57_1",
"volume": "23",
"year": "2020"
},
{
"article-title": "A comprehensive review on COVID‐19 infection and comorbidities of various organs",
"author": "Goodarzi A",
"first-page": "4",
"journal-title": "Acta Med Iranica",
"key": "e_1_2_10_58_1",
"year": "2021"
},
{
"article-title": "The effect of influenza vaccine on severity of COVID‐19 infection: an original study from Iran",
"author": "Kalantari S",
"first-page": "865",
"issue": "1",
"journal-title": "Med J Islam Repub Iran",
"key": "e_1_2_10_59_1",
"volume": "35",
"year": "2021"
},
{
"article-title": "Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: a large study from Iran with a high rate of anthracosis",
"author": "Kooranifar S",
"first-page": "481",
"issue": "1",
"journal-title": "Med J Islam Repub Iran",
"key": "e_1_2_10_60_1",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.bios.2020.112752",
"article-title": "COVID‐19 diagnosis—a review of current methods",
"author": "Yüce M",
"doi-asserted-by": "crossref",
"journal-title": "Biosens Bioelectron",
"key": "e_1_2_10_61_1",
"volume": "172",
"year": "2021"
},
{
"article-title": "Histopathologic changes and cellular events of organs systems in COVID‐19",
"author": "Nobari NN",
"first-page": "81",
"issue": "1",
"journal-title": "J Cell Mol Anesth",
"key": "e_1_2_10_62_1",
"volume": "6",
"year": "2021"
},
{
"article-title": "Obesity as an independent risk factor for COVID‐19 severity and mortality",
"author": "Tadayon Najafabadi B",
"issue": "5",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_2_10_63_1",
"volume": "5",
"year": "2023"
},
{
"article-title": "The effect of opium on severity of COVID‐19 infection: an original study from Iran",
"author": "Riahi T",
"first-page": "870",
"issue": "1",
"journal-title": "Med J Islam Repub Iran",
"key": "e_1_2_10_64_1",
"volume": "35",
"year": "2021"
}
],
"reference-count": 63,
"references-count": 63,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/iid3.1083"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Evaluation of the efficacy and safety of oral <i>N</i>‐acetylcysteine in patients with COVID‐19 receiving the routine antiviral and hydroxychloroquine protocol: A randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "11"
}