Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: An open‐label randomized controlled clinical trial
et al., Journal of Medical Virology, doi:10.1002/jmv.28393, IRCT20080901001165N55, Dec 2022
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
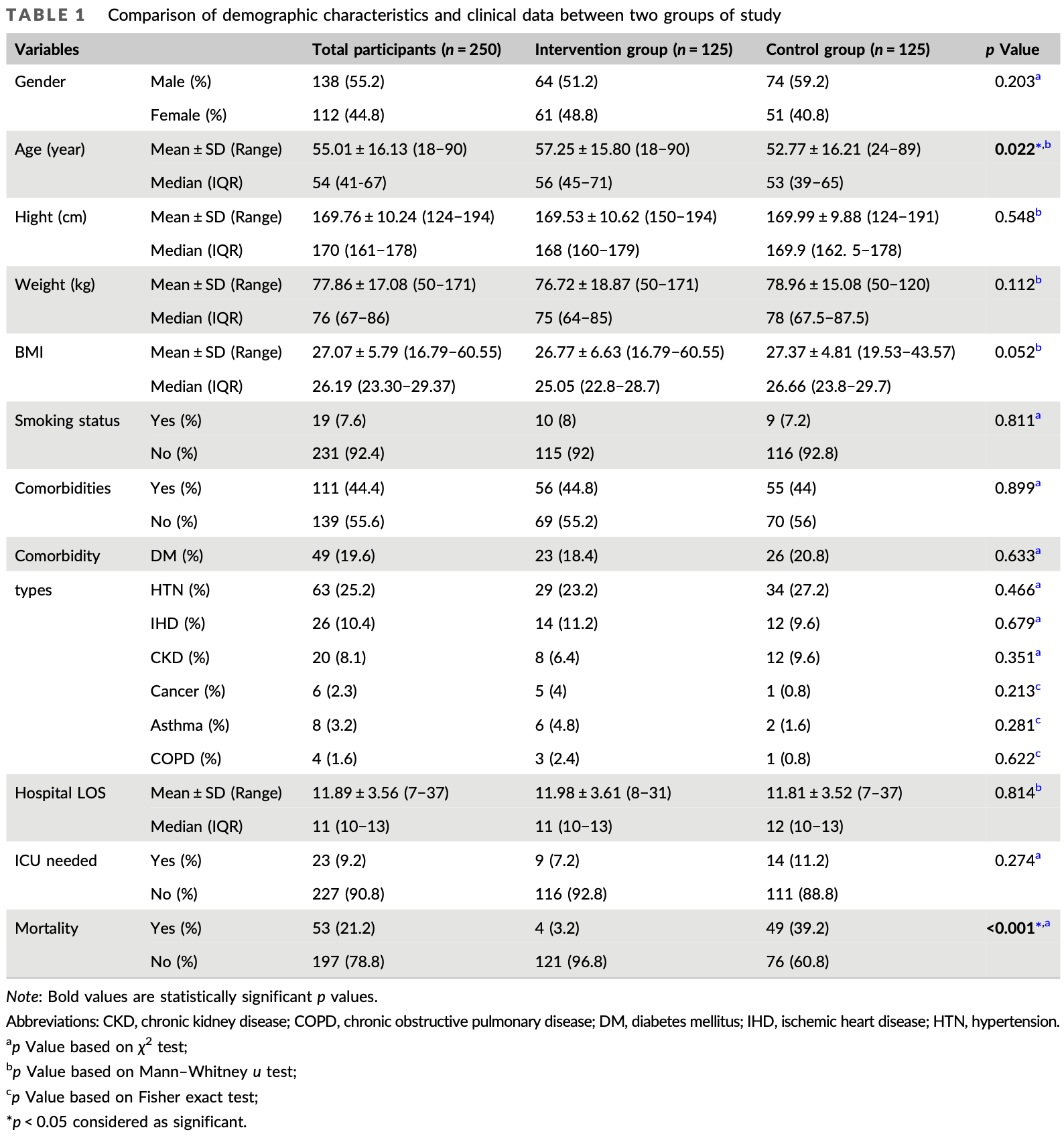
RCT 250 hospitalized COVID-19 patients showing reduced mortality rate and inflammatory markers with N-acetylcysteine (NAC) 400μg inhaled spray twice daily for 7 days as adjunctive treatment. There was no significant difference in hospital length of stay or ICU admission. The NAC group was older on average, while the control group had significantly lower SpO2 at baseline. 400 μg/day NAC inhaler spray for 7 days.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
This study is excluded in the after exclusion results of meta-analysis:
large difference in mortality vs. ICU results, significant baseline differences.
|
risk of death, 91.8% lower, RR 0.08, p < 0.001, treatment 4 of 125 (3.2%), control 49 of 125 (39.2%), NNT 2.8, Inhaled.
|
|
risk of ICU admission, 35.7% lower, RR 0.64, p = 0.38, treatment 9 of 125 (7.2%), control 14 of 125 (11.2%), NNT 25, Inhaled.
|
|
hospitalization time, 0.8% lower, relative time 0.99, p = 0.81, treatment 125, control 125, Inhaled.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Panahi et al., 19 Dec 2022, Randomized Controlled Trial, Iran, peer-reviewed, 7 authors, study period May 2021 - August 2021, trial IRCT20080901001165N55.
Contact: amirvahedian63@gmail.com, amir_saheb2000@yahoo.com.
Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: An open‐label randomized controlled clinical trial
Journal of Medical Virology, doi:10.1002/jmv.28393
The aim of this study was to evaluate the effect and safety of N-acetylcysteine (NAC) inhalation spray in the treatment of patients with coronavirus disease 2019 (COVID-19). This randomized controlled clinical trial study was conducted on patients with COVID-19. Eligible patients (n = 250) were randomly allocated into the intervention group (routine treatment + NAC inhaler spray one puff per 12 h, for 7 days) or the control group who received routine treatment alone. Clinical features, hemodynamic, hematological, biochemical parameters and patient outcomes were assessed and compared before and after treatment. The mortality rate was significantly higher in the control group than in the intervention group (39.2% vs. 3.2%, p < 0.001). Significant differences were found between the two groups (intervention and control, respectively) for white blood cell count (6.2 vs. 7.8, p < 0.001), hemoglobin (12.3 vs. 13.3, p = 0.002), C-reactive protein (CRP: 6 vs. 11.5, p < 0.0001) and aspartate aminotransferase (AST: 32 vs. 25.5, p < 0.0001). No differences were seen for hospital length of stay (11.98 ± 3.61 vs. 11.81 ± 3.52, p = 0.814) or the requirement for intensive care unit (ICU) admission (7.2% vs. 11.2%, p = 0.274). NAC was beneficial in reducing the mortality rate in patients with COVID-19 and inflammatory parameters, and a reduction in the development of severe respiratory failure; however, it did not affect the length of hospital stay or the need for ICU admission. Data on the effectiveness of NAC for Severe Acute Respiratory Syndrome Coronavirus-2 is limited and further research is required.
administration of NAC, and no information was available on its inhalation. Until now, inhalation administration of NAC has generally been associated with mucolytic activity, in contrast to oral administration of NAC, which is mainly associated with antioxidant activity. This molecule, as well as other thiol derivatives, acts primarily on the lower respiratory tract to loosen mucus, as the main target of the drug is mucin. However, some studies have shown that inhaled NAC is also effective on oxidative stress and that patients with higher oxidative stress may be good responders to inhaled NAC therapy, as the glutathione replenished by inhaled NAC can reverse the oxidant-antioxidant imbalance. 37, 42 In accord with the current study, a multi-center, prospective cohort study by Assimakopoulos et al. 43 in 2021 was conducted on hospitalized patients with moderate or severe COVID-19: patients who received standard of care were compared with patients who additionally received oral NAC (600 mg) for 14 days. The results showed that oral NAC administration (1200 mg/day) in patients with COVID-19 pneumonia reduces the risk for mechanical ventilation (MV) and 14-day and 28-day mortality. In addition, NAC improved the PO 2 /FiO 2 ratio over time and decreased WBC, CRP, D-dimer and lactic acid dehydrogenase (LDH) levels. A randomized study by Gaynitdinova et al. 44 invasive MV use between the intervention and control groups. 46 In addition, a double-blind, placebo-controlled,..
References
Aleem, Samad, Slenker, Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19)
Altay, Arif, Li, Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19, Adv Sci (Weinh), doi:10.1002/advs.202101222
Assimakopoulos, Komninos, N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study, Infect Dis
Baloch, Baloch, Zheng, Pei, The coronavirus disease 2019 (COVID-19) pandemic, Tohoku J Exp Med
Beltrán-García, Osca-Verdegal, Pallardó, Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression, Antioxidants, doi:10.3390/antiox9100936
Beyerstedt, Casaro, Rangel, COVID-19: angiotensinconverting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection, Eur J Clin Microbiol Infect Dis
Boesgaard, Aldershvile, Poulsen, Christensen, Dige-Petersen et al., N-acetylcysteine inhibits angiotensin converting enzyme in vivo, J Pharmacol Exp Ther
Calverley, Rogliani, Papi, Safety of N-acetylcysteine at high doses in chronic respiratory diseases: a review, Drug Saf
Chang, Yu, Chang, Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT, Radiology
De Alencar, Moreira, Müller, Evaluation the efficacy and safety of N-acetylcysteine inhalation spray in controlling the symptoms of patients with COVID-19: an open-label randomized controlled clinical trial, Clin Infect Dis
Dhama, Sharun, Tiwari, COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics, Hum Vaccin Immunother
Dominari, Hathaway Iii, Kapasi, Bottom-up analysis of emergent properties of N-acetylcysteine as an adjuvant therapy for COVID-19, World J Virol
Fernandes, De Brito, Reis, Sato, Pereira, SARS-CoV-2 other respiratory viruses: what does oxidative stress have to do with it?, Oxid Med Cell Longev
Flora, Grassi, Carati, Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment, Eur Respir J
Gaynitdinova, Avdeev, Merzhoeva, Berikkhanov, Medvedeva et al., As a part of complex treatment of moderately severe COVID-associated pneumonia, Pul'monologiya
Gheblawi, Wang, Viveiros, Angiotensin-Converting enzyme 2: SARS-CoV-2 receptor and regulator of the Renin-Angiotensin system: celebrating the 20th anniversary of the discovery of ACE2, Circ Res
Harrison, Lin, Wang, Mechanisms of SARS-CoV-2 transmission and pathogenesis, Trends Immunol
Horowitz, Freeman, Bruzzese, Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases, Respir Med Case Rep
Hyder, Hyder, Nasir, Ndebele, Inequitable COVID-19 vaccine distribution and its effects, Bull World Health Organ
Izquierdo, Soriano, González, Use of N-Acetylcysteine at high doses as an oral treatment for patients hospitalized with COVID-19, Sci Prog
Jayaraman, Guidelines for reporting randomized controlled trials in paediatric dentistry based on the CONSORT statement, Int J Paediat Dent
Jones, NEWSDIG: the National early warning score development and implementation group, Clin Med
Kaur, Gupta, COVID-19 vaccine: a comprehensive status report, Virus Res
Lana, Lana, Rodrigues, Nebulization of glutathione and N-Acetylcysteine as an adjuvant therapy for COVID-19 onset, Adv Redox Res
Landini, Maggio, Docquier, Rossolini, Pallecchi, Effect of high N-acetylcysteine concentrations on antibiotic activity against a large collection of respiratory pathogens, Antimicrob Agents Chemother
Marco, Foti, Corsico, Where are we with the use of N-acetylcysteine as a preventive and adjuvant treatment for COVID-19?, Eur Rev Med Pharmacol Sci
Mccarty, Dinicolantonio, Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus, Prog Cardiovasc Dis
Medical, World medical association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA
Mourmouris, Tzelves, Roidi, Fotsali, COVID-19 transmission: a rapid systematic review of current knowledge, Osong Public Health Res Perspect
Mp, N-acetylcysteine as a potential treatment for COVID-19, Future Microbiol
Muralidar, Ambi, Sekaran, Krishnan, The emergence of COVID-19 as a global pandemic: understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2, Biochimie
Ni, Yang, Yang, Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19, Crit Care
Panahi, Ghanei, Hashjin, Rezaee, Sahebkar, Potential utility of N-acetylcysteine for treating mustard lung, Crit Rev Eukaryot Gene Expr, doi:10.1615/CritRevEukaryotGeneExpr.2017019740
Panahi, Ostadmohammadi, Raygan, Sharif, Sahebkar, The effects of N-acetylcysteine administration on metabolic status and serum adiponectin levels in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled trial, J Funct. Foods
Presti, Nuzzo, Mahmeed, Molecular and proinflammatory aspects of COVID-19: the impact on cardiometabolic health, Biochim Biophys Acta Mol Basis Dis, doi:10.1016/j.bbadis.2022.166559
Rahmanzade, Rahmanzadeh, Hashemian, Tabarsi, Iran's approach to COVID-19: evolving treatment protocols and ongoing clinical trials, Front Public Health
Ramos, Vela-Pérez, Ferrández, Kubik, Ivorra, Modeling the impact of SARS-CoV-2 variants and vaccines on the spread of COVID-19, Commun Nonlinear Sci Numer Simul
Saponaro, Rutigliano, Sestito, ACE2 in the era of SARS-CoV-2: controversies and novel perspectives, Front Mol Biosci
Sawal, Ahmad, Tariq, Tahir, Essar et al., Unequal distribution of COVID-19 vaccine: a looming crisis, J Med Virol
Schwalfenberg, N-acetylcysteine: a review of clinical usefulness (an old drug with new tricks, J Nutr Metab, doi:10.1155/2021/9949453
Sharafkhah, Abdolrazaghnejad, Zarinfar, Mohammadbeigi, Massoudifar et al., Safety and efficacy of N-acetylcysteine for prophylaxis of ventilator-associated pneumonia: a randomized, double blind, placebo-controlled clinical trial, Med Gas Res
Singh, Parveen, Yadav, SARS-CoV-2: emergence of new variants and effectiveness of vaccines, Front Cell Infect Microbiol
Taher, Lashgari, Sedighi, Rahimi-Bashar, Poorolajal et al., A pilot study on intravenous N-Acetylcysteine treatment in patients with mild-to-moderate COVID19-associated acute respiratory distress syndrome, Pharmacol Rep
Ullian, Gelasco, Fitzgibbon, Beck, Morinelli, N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells, J Am Soc Nephrol
Wong, Lee, Kua, N-Acetylcysteine as adjuvant therapy for COVID-19-a perspective on the current state of the evidence, J Inflamm Res
Wu, Wang, Kuo, An update on current therapeutic drugs treating COVID-19, Curr Pharmacol Rep
Zhang, Ju, Ma, Wang, N-acetylcysteine improves oxidative stress and inflammatory response in patients with community acquired pneumonia: a randomized controlled trial, Medicine
DOI record:
{
"DOI": "10.1002/jmv.28393",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.28393",
"alternative-id": [
"10.1002/jmv.28393"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-07-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-12-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-12-19"
}
],
"author": [
{
"affiliation": [
{
"name": "Pharmacotherapy Department, School of Pharmacy Baqiyatallah University of Medical Sciences Tehran Iran"
}
],
"family": "Panahi",
"given": "Yunes",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Chemical Injuries Center, Systems Biology and Poisoning Institute Baqiyatallah University of Medical Sciences Tehran Iran"
}
],
"family": "Ghanei",
"given": "Mostafa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chemical Injuries Center, Systems Biology and Poisoning Institute Baqiyatallah University of Medical Sciences Tehran Iran"
}
],
"family": "Rahimi",
"given": "Morteza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chemical Injuries Center, Systems Biology and Poisoning Institute Baqiyatallah University of Medical Sciences Tehran Iran"
}
],
"family": "Samim",
"given": "Abbas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Trauma Research Center, Nursing Faculty Baqiyatallah University of Medical Sciences Tehran Iran"
}
],
"family": "Vahedian‐Azimi",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Postgraduate Studies and Research RCSI Medical University of Bahrain Busaiteen Kingdom of Bahrain"
}
],
"family": "Atkin",
"given": "Stephen L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8656-1444",
"affiliation": [
{
"name": "Applied Biomedical Research Center Mashhad University of Medical Sciences Mashhad Iran"
},
{
"name": "Biotechnology Research Center, Pharmaceutical Technology Institute Mashhad University of Medical Sciences Mashhad Iran"
},
{
"name": "Department of Biotechnology, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"authenticated-orcid": false,
"family": "Sahebkar",
"given": "Amirhossein",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
10
]
],
"date-time": "2022-12-10T12:24:22Z",
"timestamp": 1670675062000
},
"deposited": {
"date-parts": [
[
2023,
2,
18
]
],
"date-time": "2023-02-18T18:43:14Z",
"timestamp": 1676745794000
},
"indexed": {
"date-parts": [
[
2023,
2,
19
]
],
"date-time": "2023-02-19T05:21:40Z",
"timestamp": 1676784100167
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
12,
19
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T00:00:00Z",
"timestamp": 1671408000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T00:00:00Z",
"timestamp": 1671408000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28393",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jmv.28393",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.28393",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
12,
19
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
19
]
]
},
"published-print": {
"date-parts": [
[
2023,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.biochi.2020.09.018",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.1620/tjem.250.271",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.24171/j.phrp.2021.12.2.02",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1007/s40495-020-00216-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1016/j.virusres.2020.198114",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1080/21645515.2020.1735227",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.3389/fcimb.2021.777212",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"author": "Aleem A",
"key": "e_1_2_11_9_1",
"volume-title": "StatPearls",
"year": "2022"
},
{
"DOI": "10.1016/j.cnsns.2021.105937",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.2471/BLT.21.285616",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1002/jmv.27031",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/j.it.2020.10.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1186/s13054-020-03120-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1007/s10096-020-04138-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1155/2020/8844280",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.3389/fmolb.2020.588618",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317015",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1681/ASN.2004060458",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"article-title": "N‐acetylcysteine inhibits angiotensin converting enzyme in vivo",
"author": "Boesgaard S",
"first-page": "1239",
"issue": "3",
"journal-title": "J Pharmacol Exp Ther",
"key": "e_1_2_11_20_1",
"volume": "265",
"year": "1993"
},
{
"DOI": "10.2217/fmb-2020-0074",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1128/AAC.01334-16",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.1155/2021/9949453",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1615/CritRevEukaryotGeneExpr.2017019740",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1016/j.jff.2022.105299",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1016/j.pcad.2020.02.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"DOI": "10.1001/jama.2013.281053",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_27_1"
},
{
"DOI": "10.1111/ipd.12733",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.3389/fpubh.2020.551889",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_29_1"
},
{
"DOI": "10.1148/radiol.2363040958",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.7861/clinmedicine.12-6-501",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_31_1"
},
{
"DOI": "10.1177/00368504221074574",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.1183/09031936.97.10071535",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"DOI": "10.1097/MD.0000000000013087",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"DOI": "10.4103/2045-9912.229599",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.3390/antiox9100936",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"DOI": "10.1016/j.bbadis.2022.166559",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_37_1"
},
{
"DOI": "10.1016/j.arres.2021.100015",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1016/j.rmcr.2020.101063",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.2147/JIR.S306849",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_40_1"
},
{
"DOI": "10.5501/wjv.v10.i2.34",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_41_1"
},
{
"article-title": "Where are we with the use of N‐acetylcysteine as a preventive and adjuvant treatment for COVID‐19?",
"author": "Di Marco F",
"first-page": "715",
"issue": "2",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "e_1_2_11_42_1",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1007/s40264-020-01026-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_43_1"
},
{
"DOI": "10.1080/23744235.2021.1945675",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"article-title": "As a part of complex treatment of moderately severe COVID‐associated pneumonia",
"author": "Gaynitdinova VV",
"first-page": "21",
"issue": "1",
"journal-title": "Pul'monologiya",
"key": "e_1_2_11_45_1",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1002/advs.202101222",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1007/s43440-021-00296-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"DOI": "10.1093/cid/ciaa1443",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_48_1"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.28393"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: An open‐label randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "95"
}
