Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea
et al., medRxiv, doi:10.1101/2020.05.04.20089904, May 2020
16th treatment shown to reduce risk in
February 2021, now with p = 0.0000032 from 25 studies, recognized in 3 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
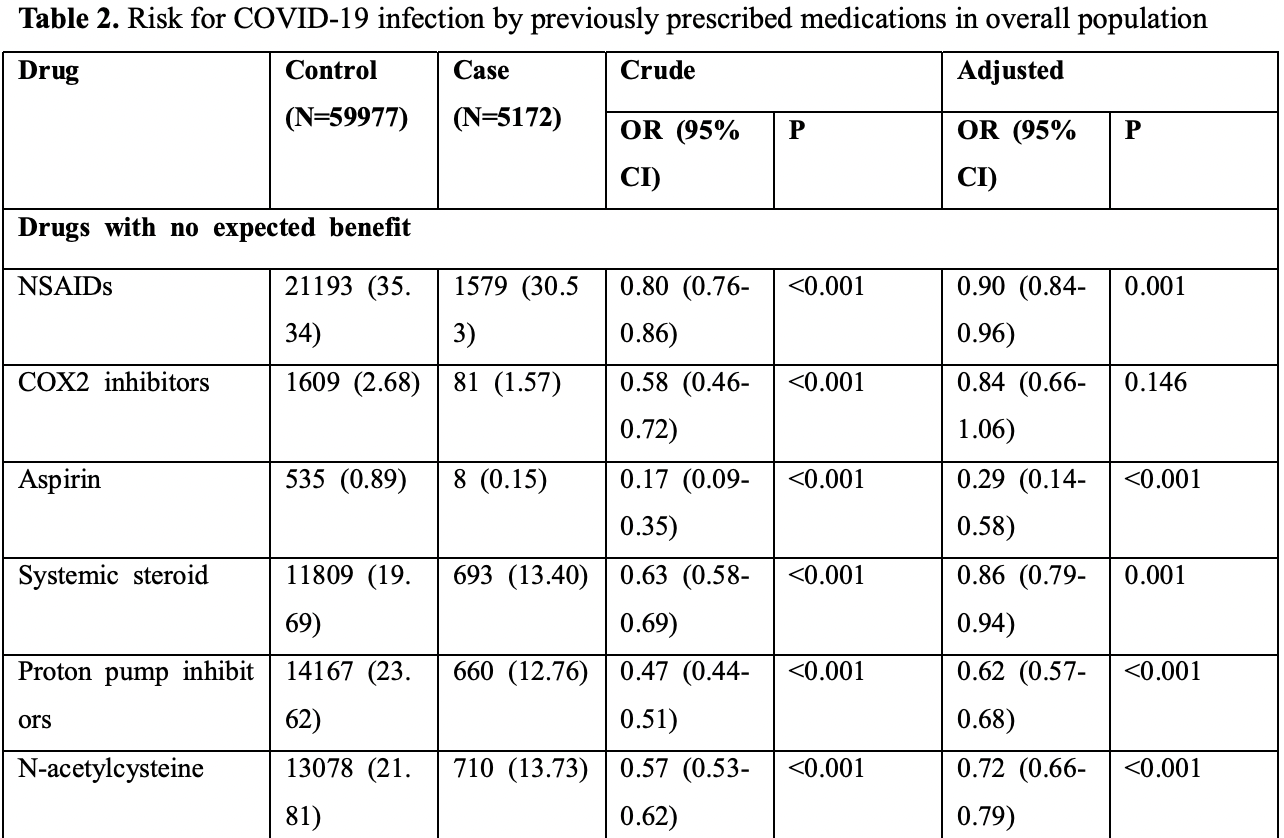
Retrospective database analysis of 65,149 in South Korea, showing significantly lower cases with existing N-acetylcysteine treatment. The journal version of this paper does not present the N-acetylcysteine results.
|
risk of case, 28.0% lower, OR 0.72, p < 0.001, treatment 710 of 5,172 (13.7%) cases,
13,078 of 59,977 (21.8%) controls, adjusted per study, case control OR, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Huh et al., 4 May 2020, retrospective, database analysis, South Korea, preprint, 10 authors.
Contact: eastside1st@gmail.com.
Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea
doi:10.1101/2020.05.04.20089904
Background. Identifying the association between medications taken prior to the infection of coronavirus disease (COVID-19) might be useful during the current pandemic until a proven treatment is developed. We aimed to determine whether the risk of developing COVID-19 was associated with the use of various drugs that may increase or decrease susceptibility to severe acute respiratory syndrome coronavirus 2 infection and COVID-19.
Methods and Findings: A case-control study was performed using a nationwide claims database of South Korea, where a large testing capacity has been available throughout the pandemic. Exposure was defined as the prescription of study drugs that would have been continued until ≤7 days before the testing for COVID-19. Adults were considered eligible if they were ≥18 years old and tested for COVID-19. Among the 65,149 eligible subjects (mean age, 48.3 years; 49.4% male), 5,172 (7.9%) were diagnosed with COVID-19. Hydroxychloroquine was not significantly associated with the risk of COVID-19 (adjusted odds ratio [aOR], 1.48; 95% CI, 0.95-2.31). In the overall population, lower risks of COVID-19 were associated with the use of camostat (aOR, 0.45; 95% CI, 0.20-1.02) and amiodarone (aOR, 0.54; 95% CI, 0.33-0.89), although the differences were not significant in the subgroup analyses. Angiotensin receptor blockers were also associated with a slightly increased risk of COVID-19 (aOR, 1.13; 95% CI, 1.01-1.26), which was also not observed in the subgroup analysis. The study limitations include potential bias regarding the controls' characteristics, inability to determine prescription compliance, and a lack of information regarding the severity of underlying conditions.
Conclusions. No medications were consistently associated with increased or decreased risks of COVID-19. These findings suggest that a more cautious approach is warranted for the clinical use of re-purposed drugs until the results are available from clinical trials. .
Author contributions. Drs Huh and Jung had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Huh, Ji, Jung. Acquisition, analysis or interpretation of data: Huh, Ji, Kang, Hong, Bae, Lee, Na, Choi, Gong, Jung. Drafting of the manuscript: Huh, Ji, Jung. Funding. This work was supported by grants from the Gachon University Gil Medical Center (grant nos. 2018-17 and 2019-11) . The sponsor of the study was not involved in the study design, analysis, and interpretation of data; writing of the report; or the decision to submit the study results for publication. .
References
Aimo, Baritussio, Emdin, Tascini, Amiodarone as a possible therapy for coronavirus infection, Eur J Prev Cardiol, doi:10.1177/2047487320919233
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis, doi:10.1016/0021-9681(87)90171-8
Chen Jun, Li, Liu Ping, Xu Qingnian, Lu et al., None
Cheng, Cheng, Chen, Lin, Chuang et al., Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus, Antiviral Res, doi:10.1016/j.antiviral.2014.12.011
Devaux, Rolain, Colson, Raoult, New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.105938
Fedson, Opal, Rordam, Hiding in Plain Sight: an Approach to Treating Patients with Severe COVID-19 Infection, mBio, doi:10.1128/mBio.00398-20
Ferrario, Jessup, Chappell, Averill, Brosnihan et al., Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2, Circulation, doi:10.1161/CIRCULATIONAHA.104.510461
Ferrario, Varagic, The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function, American Journal of Physiology-Renal Physiology, doi:10.1152/ajprenal.00110.2010
Gao, Tian, Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends, doi:10.5582/bst.2020.01047
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial
Glowacka, Bertram, Müller, Allen, Soilleux et al., Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response, Journal of Virology, doi:10.1128/jvi.02232-10
Gu, Xie, Li, Zhang, Lai et al., Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus, Scientific Reports, doi:10.1038/srep19840
Hattermann, Müller, Nitsche, Wendt, Mantke et al., Susceptibility of different eukaryotic cell lines to SARS-coronavirus, Arch Virol, doi:10.1007/s00705-004-0461-1
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Huh, Shin, Peck, Emergent Strategies for the Next Phase of COVID-19, Infect Chemother, doi:10.3947/ic.2020.52.1.105
Kindrachuk, Ork, Hart, Mazur, Holbrook et al., Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis, Antimicrob Agents Chemother, doi:10.1128/aac.03659-14
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nature Medicine, doi:10.1038/nm1267
Li, Moore, Vasilieva, Sui, Wong et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Magagnoli, Narendran, Pereira, Cummings, Hardin et al., Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, medRxiv, doi:10.1101/2020.04.16.20065920
Matsuyama, Nagata, Shirato, Kawase, Takeda et al., Efficient Activation of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein by the Transmembrane Protease TMPRSS2, Journal of Virology, doi:10.1128/jvi.01542-10
Molina, Delaugerre, Goff, Lima, Ponscarme et al., No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection, Médecine et Maladies Infectieuses, doi:10.1016/j.medmal.2020.03.006
Mossel, Huang, Narayanan, Makino, Tesh et al., Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication, J Virol, doi:10.1128/jvi.79.6.3846-3850.2005
Onder, Rezza, Brusaferro, Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy, JAMA, doi:10.1001/jama.2020.4683
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, doi:10.1001/jama.2020.6775
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease, A Review, doi:10.1001/jama.2020.6019
Shuli, Zhang Dandan, Zhiping, Li Tao, Shen Yinzhong et al., A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19), J Zhejiang Univ (Med Sci), doi:10.3785/j.issn.1008-9292.2020.03.03
Sommerstein, Kochen, Messerli, Gräni, Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers Have a Biphasic Effect?, J Am Heart Assoc, doi:10.1161/jaha.120.016509
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wu, Mcgoogan, of 72 314 Cases From the Chinese Center for Disease Control and Prevention, doi:10.1001/jama.2020.2648
Yazdany, Kim, Use of Hydroxychloroquine and Chloroquine During the COVID-19
Zhang, Zhu, Cai, Lei, Qin et al., Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19, Circ Res, doi:10.1161/circresaha.120.317134
DOI record:
{
"DOI": "10.1101/2020.05.04.20089904",
"URL": "http://dx.doi.org/10.1101/2020.05.04.20089904",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Identifying the association between medications taken prior to the infection of coronavirus disease (COVID-19) might be useful during the current pandemic until a proven treatment is developed. We aimed to determine whether the risk of developing COVID-19 was associated with the use of various drugs that may increase or decrease susceptibility to severe acute respiratory syndrome coronavirus 2 infection and COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods and Findings</jats:title><jats:p>A case-control study was performed using a nationwide claims database of South Korea, where a large testing capacity has been available throughout the pandemic. Exposure was defined as the prescription of study drugs that would have been continued until ≤7 days before the testing for COVID-19. Adults were considered eligible if they were ≥18 years old and tested for COVID-19. Among the 65,149 eligible subjects (mean age, 48.3 years; 49.4% male), 5,172 (7.9%) were diagnosed with COVID-19. Hydroxychloroquine was not significantly associated with the risk of COVID-19 (adjusted odds ratio [aOR], 1.48; 95% CI, 0.95–2.31). In the overall population, lower risks of COVID-19 were associated with the use of camostat (aOR, 0.45; 95% CI, 0.20–1.02) and amiodarone (aOR, 0.54; 95% CI, 0.33–0.89), although the differences were not significant in the subgroup analyses. Angiotensin receptor blockers were also associated with a slightly increased risk of COVID-19 (aOR, 1.13; 95% CI, 1.01–1.26), which was also not observed in the subgroup analysis. The study limitations include potential bias regarding the controls’ characteristics, inability to determine prescription compliance, and a lack of information regarding the severity of underlying conditions.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>No medications were consistently associated with increased or decreased risks of COVID-19. These findings suggest that a more cautious approach is warranted for the clinical use of re-purposed drugs until the results are available from clinical trials.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
5,
18
]
]
},
"author": [
{
"affiliation": [],
"family": "Huh",
"given": "Kyungmin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ji",
"given": "Wonjun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Minsun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Jinwook",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bae",
"given": "Gi Hwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Rugyeom",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Na",
"given": "Yewon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Hyoseon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gong",
"given": "Seon Yeong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jung",
"given": "Jaehun",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
5,
8
]
],
"date-time": "2020-05-08T14:35:27Z",
"timestamp": 1588948527000
},
"deposited": {
"date-parts": [
[
2021,
1,
14
]
],
"date-time": "2021-01-14T01:52:13Z",
"timestamp": 1610589133000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T23:32:25Z",
"timestamp": 1712273545854
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 12,
"issued": {
"date-parts": [
[
2020,
5,
8
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.05.04.20089904",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
5,
8
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
5,
8
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.3947/ic.2020.52.1.105",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.1"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.2"
},
{
"DOI": "10.1001/jama.2020.4683",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.3"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.4"
},
{
"DOI": "10.1001/jama.2020.6019",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.5"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.6"
},
{
"DOI": "10.1161/CIRCULATIONAHA.104.510461",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.7"
},
{
"DOI": "10.1152/ajprenal.00110.2010",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.8"
},
{
"DOI": "10.1161/JAHA.120.016509",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.9"
},
{
"key": "2021011110595395000_2020.05.04.20089904v2.10",
"unstructured": "Korea Centers for Disease Control and Prevention. Updates on COVID-19 in Republic of Korea (25 March 2020). Osong, Republic of Korea: 2020."
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.11"
},
{
"DOI": "10.1038/nature02145",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.12"
},
{
"DOI": "10.1128/JVI.02232-10",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.13"
},
{
"DOI": "10.1128/JVI.01542-10",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.14"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.15"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105938",
"doi-asserted-by": "crossref",
"key": "2021011110595395000_2020.05.04.20089904v2.16",
"unstructured": "Devaux CA , Rolain J-M , Colson P , Raoult D . New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? International Journal of Antimicrobial Agents. 2020:105938. doi: https://doi.org/10.1016/j.ijantimicag.2020.105938."
},
{
"DOI": "10.5582/bst.2020.01047",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.17"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.18"
},
{
"DOI": "10.3785/j.issn.1008-9292.2020.03.03",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.19"
},
{
"DOI": "10.1016/j.medmal.2020.03.006",
"doi-asserted-by": "crossref",
"key": "2021011110595395000_2020.05.04.20089904v2.20",
"unstructured": "Molina JM , Delaugerre C , Le Goff J , Mela-Lima B , Ponscarme D , Goldwirt L , et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Médecine et Maladies Infectieuses. 2020. doi: https://doi.org/10.1016/j.medmal.2020.03.006."
},
{
"DOI": "10.1101/2020.04.16.20065920",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.21"
},
{
"DOI": "10.7326/m20-1334",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.22"
},
{
"DOI": "10.1128/mBio.00398-20",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.23"
},
{
"DOI": "10.1128/AAC.03659-14",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.24"
},
{
"DOI": "10.1016/j.antiviral.2014.12.011",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.25"
},
{
"DOI": "10.1177/2047487320919233",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.26"
},
{
"key": "2021011110595395000_2020.05.04.20089904v2.27",
"unstructured": "HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19 [25 Apr 2020]. Available from: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19."
},
{
"DOI": "10.1007/s00705-004-0461-1",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.28"
},
{
"DOI": "10.1128/JVI.79.6.3846-3850.2005",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.29"
},
{
"DOI": "10.1038/nm1267",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.30"
},
{
"DOI": "10.1038/srep19840",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.31"
},
{
"key": "2021011110595395000_2020.05.04.20089904v2.32",
"unstructured": "Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers [25 Apr 2020]. Available from: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang."
},
{
"DOI": "10.1161/circresaha.120.317134",
"doi-asserted-by": "publisher",
"key": "2021011110595395000_2020.05.04.20089904v2.33"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.05.04.20089904"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Association of previous medications with the risk of COVID-19: a nationwide claims-based study from South Korea",
"type": "posted-content"
}
huh
